
PDF Publication Title:
Text from PDF Page: 018
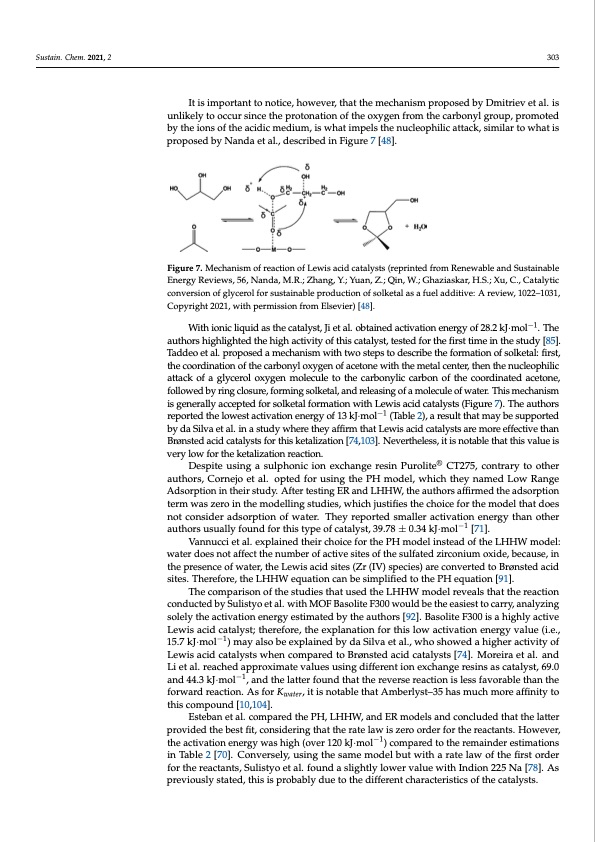
is proposed by Nanda et al., described in Figure 7 [48]. With ionic liquid as the catalyst, Ji et al. obtained activation energy of 28.2 kJ· mol−1. The authors highlighted the high activity of this catalyst, tested for the first time in the study [85]. Taddeo et al. proposed a mechanism with two steps to describe the formation of solketal: first, the coordination of the carbonyl oxygen of acetone with the metal center, Sustain. Chem. 2021, 2 303 then the nucleophilic attack of a glycerol oxygen molecule to the carbonylic carbon of the coordinated acetone, followed by ring closure, forming solketal, and releasing of a mole- cule of water. This mechanism is generally accepted for solketal formation with Lewis acid It is important to notice, however, that the mechanism proposed by Dmitrie−1v et al. is catalysts (Figure 7). The authors reported the lowest activation energy of 13 kJ·mol (Table 2), unlikely to occur since the protonation of the oxygen from the carbonyl group, promoted a result that may be supported by da Silva et al. in a study where they affirm that Lewis acid by the ions of the acidic medium, is what impels the nucleophilic attack, similar to what is catalysts are more effective than Brønsted acid catalysts for this ketalization [74,103]. Never- proposed by Nanda et al., described in Figure 7 [48]. theless, it is notable that this value is very low for the ketalization reaction. Figure 7. Mechanism of reaction of Lewis acid catalysts (reprinted from Renewable and Sustainable Energy Reviews, 56, Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C., Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review, 1022–1031, Copyright 2021, with permission from Elsevier) [48]. With ionic liquid as the catalyst, Ji et al. obtained activation energy of 28.2 kJ·mol−1. The authors highlighted the high activity of this catalyst, tested for the first time in the study [85]. Taddeo et al. proposed a mechanism with two steps to describe the formation of solketal: first, the coordination of the carbonyl oxygen of acetone with the metal center, then the nucleophilic attack of a glycerol oxygen molecule to the carbonylic carbon of the coordinated acetone, followed by ring closure, forming solketal, and releasing of a molecule of water. This mechanism is generally accepted for solketal formation with Lewis acid catalysts (Figure 7). The authors reported the lowest activation energy of 13 kJ·mol−1 (Table 2), a result that may be supported by da Silva et al. in a study where they affirm that Lewis acid catalysts are more effective than Brønsted acid catalysts for this ketalization [74,103]. Nevertheless, it is notable that this value is very low for the ketalization reaction. Despite using a sulphonic ion exchange resin Purolite® CT275, contrary to other authors, Cornejo et al. opted for using the PH model, which they named Low Range Adsorption in their study. After testing ER and LHHW, the authors affirmed the adsorption term was zero in the modelling studies, which justifies the choice for the model that does not consider adsorption of water. They reported smaller activation energy than other authors usually found for this type of catalyst, 39.78 ± 0.34 kJ·mol−1 [71]. Vannucci et al. explained their choice for the PH model instead of the LHHW model: water does not affect the number of active sites of the sulfated zirconium oxide, because, in the presence of water, the Lewis acid sites (Zr (IV) species) are converted to Brønsted acid sites. Therefore, the LHHW equation can be simplified to the PH equation [91]. The comparison of the studies that used the LHHW model reveals that the reaction conducted by Sulistyo et al. with MOF Basolite F300 would be the easiest to carry, analyzing solely the activation energy estimated by the authors [92]. Basolite F300 is a highly active Lewis acid catalyst; therefore, the explanation for this low activation energy value (i.e., 15.7 kJ·mol−1) may also be explained by da Silva et al., who showed a higher activity of Lewis acid catalysts when compared to Brønsted acid catalysts [74]. Moreira et al. and Li et al. reached approximate values using different ion exchange resins as catalyst, 69.0 and 44.3 kJ·mol−1, and the latter found that the reverse reaction is less favorable than the forward reaction. As for Kwater, it is notable that Amberlyst–35 has much more affinity to this compound [10,104]. Esteban et al. compared the PH, LHHW, and ER models and concluded that the latter provided the best fit, considering that the rate law is zero order for the reactants. However, the activation energy was high (over 120 kJ·mol−1) compared to the remainder estimations in Table 2 [70]. Conversely, using the same model but with a rate law of the first order for the reactants, Sulistyo et al. found a slightly lower value with Indion 225 Na [78]. As previously stated, this is probably due to the different characteristics of the catalysts.PDF Image | Continuous Valorization of Glycerol into Solketal
PDF Search Title:
Continuous Valorization of Glycerol into SolketalOriginal File Name Searched:
suschem-02-00017.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |