
PDF Publication Title:
Text from PDF Page: 004
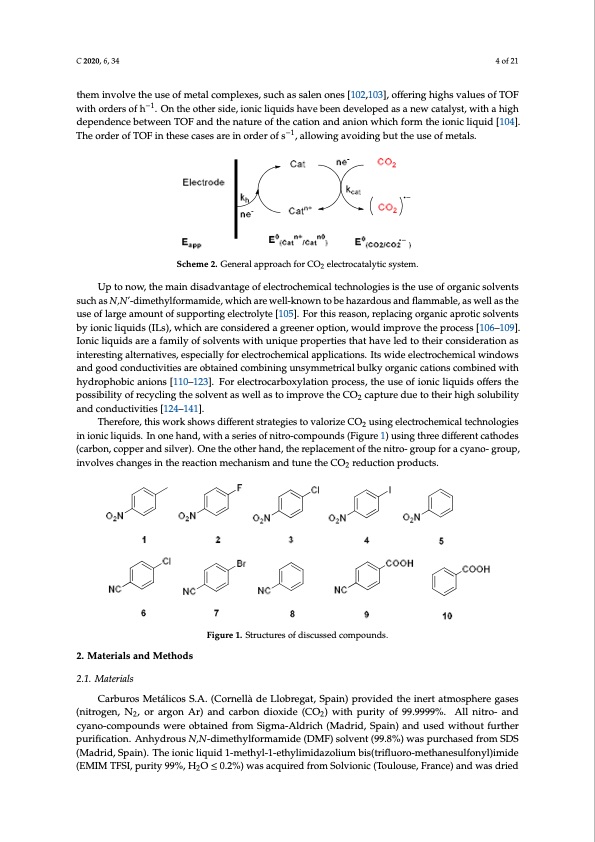
to provide CO. Koper and co-workers proposed a different mechanism [99], where the formation of the CO2−, anion intermediate, will be protonated yielding to [Co(P)–(COOH)]0 intermediate. Then, [Co(P)–(COOH)]0 will decompose to CO. Finally, Yao et al. proposed a pre-activation process to form a local proton source could facilitate CO2 reduction [100]. The electrocatalysts participates in an electron transfer reaction (on the electrode surface) and C 2020, 6, 34 4 of 21 facilitates acceleration of a chemical reaction. Both electron transfer and chemical kinetics must be fast for an efficient electrocatalyst. In addition, an optimal electrocatalyst must display a good ththeemrminovdoylnvaemthicemusaetcohfbmeetwtaelecnomthpelerxeedso,xsupcohteanstisaalle(En0o)nfoers[t1h0e2e,1le0c3t]r,oonffetrainsgfehrigrheascvtiaoluneasnodfTthOeF −1 wchitehmoircdaelrsreoafchtion. Othnathies obtehienrgsicdaeta, liyozneicdli(qinuidthsishawvoerbke;eCnOd2evreldoupcetdioans).aCnhewemciactallytustn, iwngithoaf hthigeh deelepcetnrodceantacelybstetcwaneeonptTimOiFzeanthdetsheefancatotursr.eAofgtehneercaltiaopnparonadchanfioornanwehliecchtrfocramtaltyhteiciosynsictelmiquisidgi[v1e0n4]. TihneSochrdeemreo2f.TOF in these cases are in order of s−1, allowing avoiding but the use of metals. C 2020, 6, x FOR PEER REVIEW 4 of 21 is lost due to the chemical reaction between reagent and electrocatalyst. Electrocatalyst offer critical solutions to lowering the overpotentials, improving selectivity, and increasing the reaction kinetics of carbon dioxide conversion [101]. There are a lot of different catalysts reported in literature, although most of them involve the use of metal complexes, such as salen ones [102,103], offering highs values of TOF with orders of h−1. On the other side, ionic liquids have been developed as a new catalyst, with a high dependence between TOF and the nature of the cation and anion which form the ionic liquid [104]. The order of TOF in these cases are in order of s−1, allowing avoiding but the Scheme2..GeneralapproachforCO2 eelelecctrtrooccaatatalylytitcicssyysstetemm.. 2 use of metals. Up to now, the main disadvantage of electrochemical technologies is the use of organic solvents Up to now, the main disadvantage of electrochemical technologies is the use of organic solvents The electrocatalytic activity can be analyzed in terms of in cyclic voltammetry (CV). In a CV such as N,N’-dimethylformamide, which are well-known to be hazardous and flammable, as well as such as N,N’-dimethylformamide, which are well-known to be hazardous and flammable, as well as the under a dry inert atmosphere, an electrocatalyst should show a reversible redox couple. Upon the use of large amount of supporting electrolyte [105]. For this reason, replacing organic aprotic use of large amount of supporting electrolyte [105]. For this reason, replacing organic aprotic solvents addition of the reagent which is catalyzed (i.e., CO2), the diffusion limited current should increase solvents by ionic liquids (ILs), which are considered a greener option, would improve the process bsyiginoinficalniqtluyi,dwsh(IiLlest)h, we phoictehnatiraelcsohnifstisdaenreodiacagllrye,eannedr othpetiroenv,ewrsoibuildityiminptrhoeverethuernporoxcidesasti[o1n06w–a1v0e9]. [106–109]. Ionic liquids are a family of solvents with unique properties that have led to their Ionic liquids are a family of solvents with unique properties that have led to their consideration as consideration as interesting alternatives, especially for electrochemical applications. Its wide interesting alternatives, especially for electrochemical applications. Its wide electrochemical windows electrochemical windows and good conductivities are obtained combining unsymmetrical bulky and good conductivities are obtained combining unsymmetrical bulky organic cations combined with organic cations combined with hydrophobic anions [110–123]. For electrocarboxylation process, the hydrophobic anions [110–123]. For electrocarboxylation process, the use of ionic liquids offers the use of ionic liquids offers the possibility of recycling the solvent as well as to improve the CO2 capture possibility of recycling the solvent as well as to improve the CO2 capture due to their high solubility due to their high solubility and conductivities [124–141]. and conductivities [124–141]. Therefore, this work shows different strategies to valorize CO2 using electrochemical Therefore,thisworkshowsdifferentstrategiestovalorizeCO usingelectrochemicaltechnologies 2 technologies in ionic liquids. In one hand, with a series of nitro-compounds (Figure 1) using three in ionic liquids. In one hand, with a series of nitro-compounds (Figure 1) using three different cathodes different cathodes (carbon, copper and silver). One the other hand, the replacement of the nitro- (carbon, copper and silver). One the other hand, the replacement of the nitro- group for a cyano- group, group for a cyano- group, involves changes in the reaction mechanism and tune the CO2 reduction involveschangesinthereactionmechanismandtunetheCO reductionproducts. products. 2 2. Materials and Methods 2. Materials and Methods 2.1. Materials 2.1. Materials Figure 1. Structures of discussed compounds. Figure 1. Structures of discussed compounds. Carburos Metálicos S.A. (Cornellà de Llobregat, Spain) provided the inert atmosphere gases Carburos Metálicos S.A. (Cornellà de Llobregat, Spain) provided the inert atmosphere gases (nitrogen, N2, or argon Ar) and carbon dioxide (CO2) with purity of 99.9999%. All nitro- and (nitrogen, N2, or argon Ar) and carbon dioxide (CO2) with purity of 99.9999%. All nitro- and cyano- cyano-compounds were obtained from Sigma-Aldrich (Madrid, Spain) and used without further compounds were obtained from Sigma-Aldrich (Madrid, Spain) and used without further purification. Anhydrous N,N-dimethylformamide (DMF) solvent (99.8%) was purchased from SDS purification. Anhydrous N,N-dimethylformamide (DMF) solvent (99.8%) was purchased from SDS (Madrid, Spain). The ionic liquid 1-methyl-1-ethylimidazolium bis(trifluoro-methanesulfonyl)imide (Madrid, Spain). The ionic liquid 1-methyl-1-ethylimidazolium bis(trifluoro-methanesulfonyl)imide (EMIM TFSI, purity 99%, H2O ≤ 0.2%) was acquired from Solvionic (Toulouse, France) and was dried (EMIM TFSI, purity 99%, H2O ≤ 0.2%) was acquired from Solvionic (Toulouse, France) and was dried under vacuum using activated molecular sieves for 24 h to make sure that the amount of water was always less than H2O ≤ 0.001% [142].PDF Image | Electrochemical Tuning of CO2 Reactivity in Ionic Liquids
PDF Search Title:
Electrochemical Tuning of CO2 Reactivity in Ionic LiquidsOriginal File Name Searched:
carbon-06-00034.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |