
PDF Publication Title:
Text from PDF Page: 004
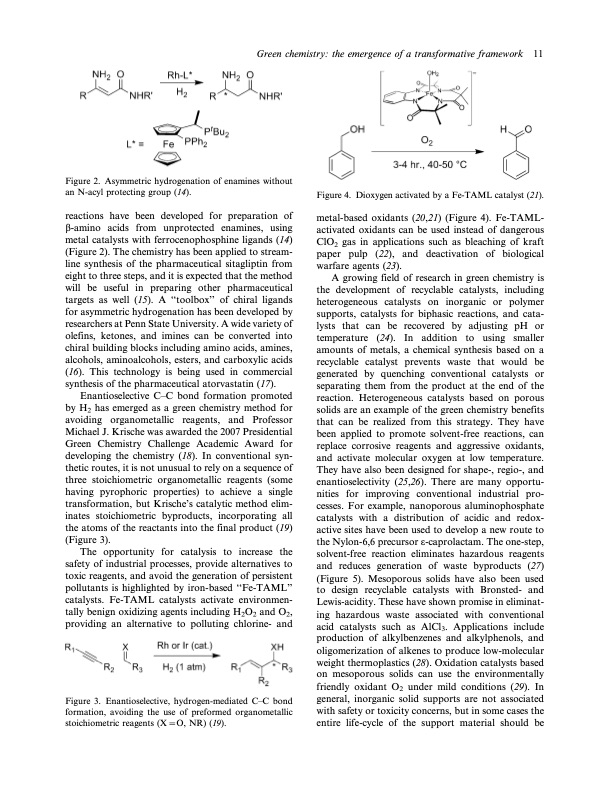
Green chemistry: the emergence of a transformative framework 11 Figure 2. Asymmetric hydrogenation of enamines without an N-acyl protecting group (14). reactions have been developed for preparation of b-amino acids from unprotected enamines, using metal catalysts with ferrocenophosphine ligands (14) (Figure 2). The chemistry has been applied to stream- line synthesis of the pharmaceutical sitagliptin from eight to three steps, and it is expected that the method will be useful in preparing other pharmaceutical targets as well (15). A ‘‘toolbox’’ of chiral ligands for asymmetric hydrogenation has been developed by researchers at Penn State University. A wide variety of olefins, ketones, and imines can be converted into chiral building blocks including amino acids, amines, alcohols, aminoalcohols, esters, and carboxylic acids (16). This technology is being used in commercial synthesis of the pharmaceutical atorvastatin (17). Enantioselective CC bond formation promoted by H2 has emerged as a green chemistry method for avoiding organometallic reagents, and Professor Michael J. Krische was awarded the 2007 Presidential Green Chemistry Challenge Academic Award for developing the chemistry (18). In conventional syn- thetic routes, it is not unusual to rely on a sequence of three stoichiometric organometallic reagents (some having pyrophoric properties) to achieve a single transformation, but Krische’s catalytic method elim- inates stoichiometric byproducts, incorporating all the atoms of the reactants into the final product (19) (Figure 3). The opportunity for catalysis to increase the safety of industrial processes, provide alternatives to toxic reagents, and avoid the generation of persistent pollutants is highlighted by iron-based ‘‘Fe-TAML’’ catalysts. Fe-TAML catalysts activate environmen- tally benign oxidizing agents including H2O2 and O2, providing an alternative to polluting chlorine- and Figure 3. Enantioselective, hydrogen-mediated CC bond formation, avoiding the use of preformed organometallic stoichiometric reagents (XO, NR) (19). Figure 4. Dioxygen activated by a Fe-TAML catalyst (21). metal-based oxidants (20,21) (Figure 4). Fe-TAML- activated oxidants can be used instead of dangerous ClO2 gas in applications such as bleaching of kraft paper pulp (22), and deactivation of biological warfare agents (23). A growing field of research in green chemistry is the development of recyclable catalysts, including heterogeneous catalysts on inorganic or polymer supports, catalysts for biphasic reactions, and cata- lysts that can be recovered by adjusting pH or temperature (24). In addition to using smaller amounts of metals, a chemical synthesis based on a recyclable catalyst prevents waste that would be generated by quenching conventional catalysts or separating them from the product at the end of the reaction. Heterogeneous catalysts based on porous solids are an example of the green chemistry benefits that can be realized from this strategy. They have been applied to promote solvent-free reactions, can replace corrosive reagents and aggressive oxidants, and activate molecular oxygen at low temperature. They have also been designed for shape-, regio-, and enantioselectivity (25,26). There are many opportu- nities for improving conventional industrial pro- cesses. For example, nanoporous aluminophosphate catalysts with a distribution of acidic and redox- active sites have been used to develop a new route to the Nylon-6,6 precursor o-caprolactam. The one-step, solvent-free reaction eliminates hazardous reagents and reduces generation of waste byproducts (27) (Figure 5). Mesoporous solids have also been used to design recyclable catalysts with Bronsted- and Lewis-acidity. These have shown promise in eliminat- ing hazardous waste associated with conventional acid catalysts such as AlCl3. Applications include production of alkylbenzenes and alkylphenols, and oligomerization of alkenes to produce low-molecular weight thermoplastics (28). Oxidation catalysts based on mesoporous solids can use the environmentally friendly oxidant O2 under mild conditions (29). In general, inorganic solid supports are not associated with safety or toxicity concerns, but in some cases the entire life-cycle of the support material should bePDF Image | Green chemistry: the emergence of a transformative framework
PDF Search Title:
Green chemistry: the emergence of a transformative frameworkOriginal File Name Searched:
green-chemistry.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |