
PDF Publication Title:
Text from PDF Page: 017
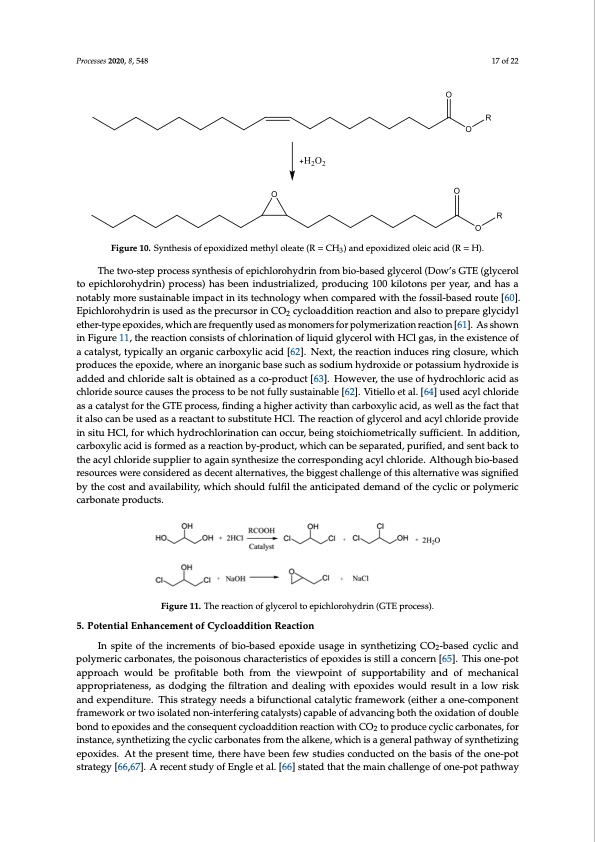
produce epoxidized methyl oleate or epoxidized oleic acid. However, it is worth noting that epoxidation is a highly exothermic reaction (as H = −55 kcal/mol for each double bond), thus H2O2 is slowly added or added by a stepwise manner in semi-batch operations, and requires a long reaction time. In order to avoid heat and mass transfer limitation, process intensification using a microreactor has been proposed by applying a novel TiO2 coated-wall microcapillary reactor [59]. The microreactor Processes 2020, 8, 548 17 of 22 could decrease the reaction time from several hours to a few minutes with higher product selectivity. FiFgiugruere101.0S.ySnynththeseisisofofepepoxoixdidiziezdedmmetehtyhlyolloelaetaete(R(R==CH3)andepoxiidiizedoleicacid(R==HH).). 3 The two-step process synthesis of epichlorohydrin from bio-based glycerol (Dow’s GTE (glycerol The two-step process synthesis of epichlorohydrin from bio-based glycerol (Dow’s GTE to epichlorohydrin) process) has been industrialized, producing 100 kilotons per year, and has a (glycerol to epichlorohydrin) process) has been industrialized, producing 100 kilotons per year, and notably more sustainable impact in its technology when compared with the fossil-based route [60]. has a notably more sustainable impact in its technology when compared with the fossil-based route EpichlorohydrinisusedastheprecursorinCO cycloadditionreactionandalsotoprepareglycidyl [60]. Epichlorohydrin is used as the precursor2in CO2 cycloaddition reaction and also to prepare ether-type epoxides, which are frequently used as monomers for polymerization reaction [61]. As shown glycidyl ether-type epoxides, which are frequently used as monomers for polymerization reaction in Figure 11, the reaction consists of chlorination of liquid glycerol with HCl gas, in the existence of [61]. As shown in Figure 11, the reaction consists of chlorination of liquid glycerol with HCl gas, in a catalyst, typically an organic carboxylic acid [62]. Next, the reaction induces ring closure, which the existence of a catalyst, typically an organic carboxylic acid [62]. Next, the reaction induces ring produces the epoxide, where an inorganic base such as sodium hydroxide or potassium hydroxide is closure, which produces the epoxide, where an inorganic base such as sodium hydroxide or aPdrodceessdesa2n02d0,c8h, xloFrOidRePEsEaRltRiEsVoIbEWtained as a co-product [63]. However, the use of hydrochloric a1c8idofa2s2 potassium hydroxide is added and chloride salt is obtained as a co-product [63]. However, the use of chloride source causes the process to be not fully sustainable [62]. Vitiello et al. [64] used acyl chloride hydrochloric acid as chloride source causes the process to be not fully sustainable [62]. Vitiello et al. as well as the fact that it also can be used as a reactant to substitute HCl. The reaction of glycerol and as a catalyst for the GTE process, finding a higher activity than carboxylic acid, as well as the fact that [64] used acyl chloride as a catalyst for the GTE process, finding a higher activity than carboxylic acid, acyl chloride provide in situ HCl, for which hydrochlorination can occur, being stoichiometrically it also can be used as a reactant to substitute HCl. The reaction of glycerol and acyl chloride provide sufficient. In addition, carboxylic acid is formed as a reaction by-product, which can be separated, in situ HCl, for which hydrochlorination can occur, being stoichiometrically sufficient. In addition, Processes 2020, 8, x; doi: FOR PEER REVIEW www.mdpi.com/journal/processes purified, and sent back to the acyl chloride supplier to again synthesize the corresponding acyl carboxylic acid is formed as a reaction by-product, which can be separated, purified, and sent back to chloride. Although bio-based resources were considered as decent alternatives, the biggest challenge the acyl chloride supplier to again synthesize the corresponding acyl chloride. Although bio-based of this alternative was signified by the cost and availability, which should fulfil the anticipated resources were considered as decent alternatives, the biggest challenge of this alternative was signified demand of the cyclic or polymeric carbonate products. by the cost and availability, which should fulfil the anticipated demand of the cyclic or polymeric carbonate products. Figure 11. The reaction of glycerol to epichlorohydrin (GTE process). Figure 11. The reaction of glycerol to epichlorohydrin (GTE process). 5. Potential Enhancement of Cycloaddition Reaction 5. Potential Enhancement of Cycloaddition Reaction In spite of the increments of bio-based epoxide usage in synthetizing CO2-based cyclic and In spite of the increments of bio-based epoxide usage in synthetizing CO2-based cyclic and polymeric carbonates, the poisonous characteristics of epoxides is still a concern [65]. This one-pot polymeric carbonates, the poisonous characteristics of epoxides is still a concern [65]. This one-pot approach would be profitable both from the viewpoint of supportability and of mechanical approach would be profitable both from the viewpoint of supportability and of mechanical appropriateness, as dodging the filtration and dealing with epoxides would result in a low risk appropriateness, as dodging the filtration and dealing with epoxides would result in a low risk and and expenditure. This strategy needs a bifunctional catalytic framework (either a one-component expenditure. This strategy needs a bifunctional catalytic framework (either a one-component framework or two isolated non-interfering catalysts) capable of advancing both the oxidation of double framework or two isolated non-interfering catalysts) capable of advancing both the oxidation of bond to epoxides and the consequent cycloaddition reaction with CO2 to produce cyclic carbonates, for double bond to epoxides and the consequent cycloaddition reaction with CO2 to produce cyclic instance, synthetizing the cyclic carbonates from the alkene, which is a general pathway of synthetizing carbonates, for instance, synthetizing the cyclic carbonates from the alkene, which is a general epoxides. At the present time, there have been few studies conducted on the basis of the one-pot pathway of synthetizing epoxides. At the present time, there have been few studies conducted on the strategy [66,67]. A recent study of Engle et al. [66] stated that the main challenge of one-pot pathway basis of the one-pot strategy [66,67]. A recent study of Engle et al. [66] stated that the main challenge of one-pot pathway to synthesize cyclic carbonate is in integrating the epoxidation and cycloaddition reaction of CO2, because a common catalyst used for a cycloaddition reaction such as TBAB deactivates a catalyst used for epoxidation reaction. The advancement of this cycloaddition reaction provides insight into utilizing CO2 directly from waste source CO such as flue gas [18]. The perspective of utilizing a waste source of carbon dioxidePDF Image | Green Pathway Utilizing CO2 Cycloaddition Reaction Epoxide
PDF Search Title:
Green Pathway Utilizing CO2 Cycloaddition Reaction EpoxideOriginal File Name Searched:
processes-08-00548.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |