
PDF Publication Title:
Text from PDF Page: 023
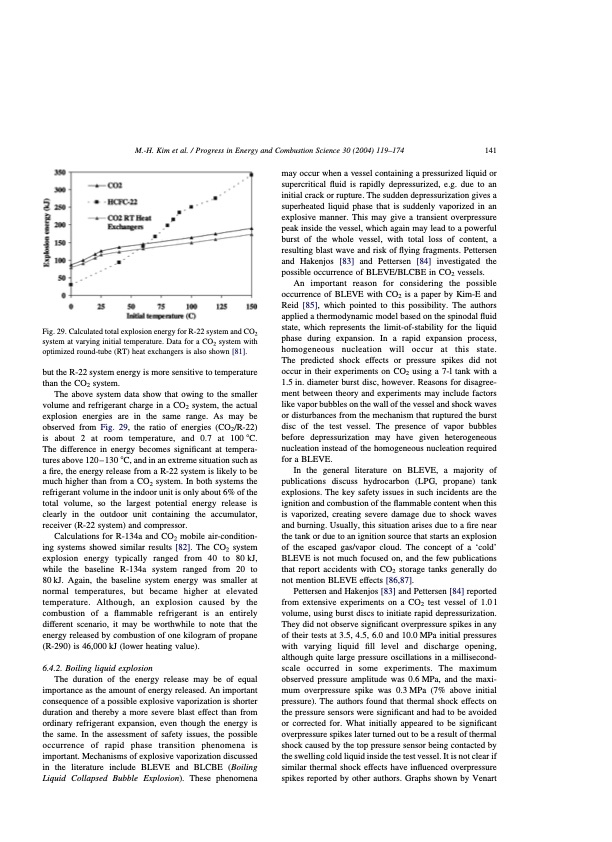
M.-H. Kim et al. / Progress in Energy and Combustion Science 30 (2004) 119–174 141 Fig. 29. Calculated total explosion energy for R-22 system and CO2 system at varying initial temperature. Data for a CO2 system with optimized round-tube (RT) heat exchangers is also shown [81]. but the R-22 system energy is more sensitive to temperature than the CO2 system. The above system data show that owing to the smaller volume and refrigerant charge in a CO2 system, the actual explosion energies are in the same range. As may be observed from Fig. 29, the ratio of energies (CO2/R-22) is about 2 at room temperature, and 0.7 at 1008C. The difference in energy becomes significant at tempera- tures above 120 – 130 8C, and in an extreme situation such as a fire, the energy release from a R-22 system is likely to be much higher than from a CO2 system. In both systems the refrigerant volume in the indoor unit is only about 6% of the total volume, so the largest potential energy release is clearly in the outdoor unit containing the accumulator, receiver (R-22 system) and compressor. Calculations for R-134a and CO2 mobile air-condition- ing systems showed similar results [82]. The CO2 system explosion energy typically ranged from 40 to 80kJ, while the baseline R-134a system ranged from 20 to 80 kJ. Again, the baseline system energy was smaller at normal temperatures, but became higher at elevated temperature. Although, an explosion caused by the combustion of a flammable refrigerant is an entirely different scenario, it may be worthwhile to note that the energy released by combustion of one kilogram of propane (R-290) is 46,000 kJ (lower heating value). 6.4.2. Boiling liquid explosion The duration of the energy release may be of equal importance as the amount of energy released. An important consequence of a possible explosive vaporization is shorter duration and thereby a more severe blast effect than from ordinary refrigerant expansion, even though the energy is the same. In the assessment of safety issues, the possible occurrence of rapid phase transition phenomena is important. Mechanisms of explosive vaporization discussed in the literature include BLEVE and BLCBE (Boiling Liquid Collapsed Bubble Explosion). These phenomena may occur when a vessel containing a pressurized liquid or supercritical fluid is rapidly depressurized, e.g. due to an initial crack or rupture. The sudden depressurization gives a superheated liquid phase that is suddenly vaporized in an explosive manner. This may give a transient overpressure peak inside the vessel, which again may lead to a powerful burst of the whole vessel, with total loss of content, a resulting blast wave and risk of flying fragments. Pettersen and Hakenjos [83] and Pettersen [84] investigated the possible occurrence of BLEVE/BLCBE in CO2 vessels. An important reason for considering the possible occurrence of BLEVE with CO2 is a paper by Kim-E and Reid [85], which pointed to this possibility. The authors applied a thermodynamic model based on the spinodal fluid state, which represents the limit-of-stability for the liquid phase during expansion. In a rapid expansion process, homogeneous nucleation will occur at this state. The predicted shock effects or pressure spikes did not occur in their experiments on CO2 using a 7-l tank with a 1.5 in. diameter burst disc, however. Reasons for disagree- ment between theory and experiments may include factors like vapor bubbles on the wall of the vessel and shock waves or disturbances from the mechanism that ruptured the burst disc of the test vessel. The presence of vapor bubbles before depressurization may have given heterogeneous nucleation instead of the homogeneous nucleation required for a BLEVE. In the general literature on BLEVE, a majority of publications discuss hydrocarbon (LPG, propane) tank explosions. The key safety issues in such incidents are the ignition and combustion of the flammable content when this is vaporized, creating severe damage due to shock waves and burning. Usually, this situation arises due to a fire near the tank or due to an ignition source that starts an explosion of the escaped gas/vapor cloud. The concept of a ‘cold’ BLEVE is not much focused on, and the few publications that report accidents with CO2 storage tanks generally do not mention BLEVE effects [86,87]. Pettersen and Hakenjos [83] and Pettersen [84] reported from extensive experiments on a CO2 test vessel of 1.0 l volume, using burst discs to initiate rapid depressurization. They did not observe significant overpressure spikes in any of their tests at 3.5, 4.5, 6.0 and 10.0 MPa initial pressures with varying liquid fill level and discharge opening, although quite large pressure oscillations in a millisecond- scale occurred in some experiments. The maximum observed pressure amplitude was 0.6 MPa, and the maxi- mum overpressure spike was 0.3 MPa (7% above initial pressure). The authors found that thermal shock effects on the pressure sensors were significant and had to be avoided or corrected for. What initially appeared to be significant overpressure spikes later turned out to be a result of thermal shock caused by the top pressure sensor being contacted by the swelling cold liquid inside the test vessel. It is not clear if similar thermal shock effects have influenced overpressure spikes reported by other authors. Graphs shown by VenartPDF Image | CO2 Vapor Compression Systems
PDF Search Title:
CO2 Vapor Compression SystemsOriginal File Name Searched:
co2-vapor-compression-systems.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |