
PDF Publication Title:
Text from PDF Page: 005
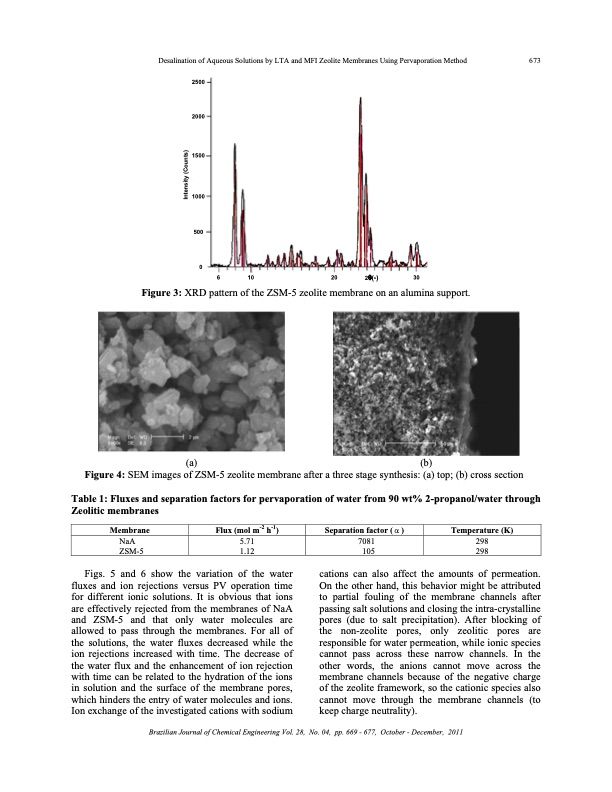
Desalination of Aqueous Solutions by LTA and MFI Zeolite Membranes Using Pervaporation Method 673 2 2 2 5 5 50 0 0 0 0 0 2000 1500 1000 500 0 2θ(°) (a) (b) Figure 4: SEM images of ZSM-5 zeolite membrane after a three stage synthesis: (a) top; (b) cross section Table 1: Fluxes and separation factors for pervaporation of water from 90 wt% 2-propanol/water through Zeolitic membranes 6 10 Figure 3: XRD pattern of the ZSM-5 zeolite membrane on an alumina support. 20 30 Membrane NaA ZSM-5 Flux (mol m-2 h-1) 5.71 1.12 Separation factor ( α ) 7081 105 Temperature (K) 298 298 Figs. 5 and 6 show the variation of the water fluxes and ion rejections versus PV operation time for different ionic solutions. It is obvious that ions are effectively rejected from the membranes of NaA and ZSM-5 and that only water molecules are allowed to pass through the membranes. For all of the solutions, the water fluxes decreased while the ion rejections increased with time. The decrease of the water flux and the enhancement of ion rejection with time can be related to the hydration of the ions in solution and the surface of the membrane pores, which hinders the entry of water molecules and ions. Ion exchange of the investigated cations with sodium cations can also affect the amounts of permeation. On the other hand, this behavior might be attributed to partial fouling of the membrane channels after passing salt solutions and closing the intra-crystalline pores (due to salt precipitation). After blocking of the non-zeolite pores, only zeolitic pores are responsible for water permeation, while ionic species cannot pass across these narrow channels. In the other words, the anions cannot move across the membrane channels because of the negative charge of the zeolite framework, so the cationic species also cannot move through the membrane channels (to keep charge neutrality). Brazilian Journal of Chemical Engineering Vol. 28, No. 04, pp. 669 - 677, October - December, 2011 Intensity (Counts)PDF Image | DESALINATION OF AQUEOUS SOLUTIONS ZEOLITE MEMBRANES
PDF Search Title:
DESALINATION OF AQUEOUS SOLUTIONS ZEOLITE MEMBRANESOriginal File Name Searched:
PCZPTXXvZ6pQgGbDbcz9VXd.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |