
PDF Publication Title:
Text from PDF Page: 004
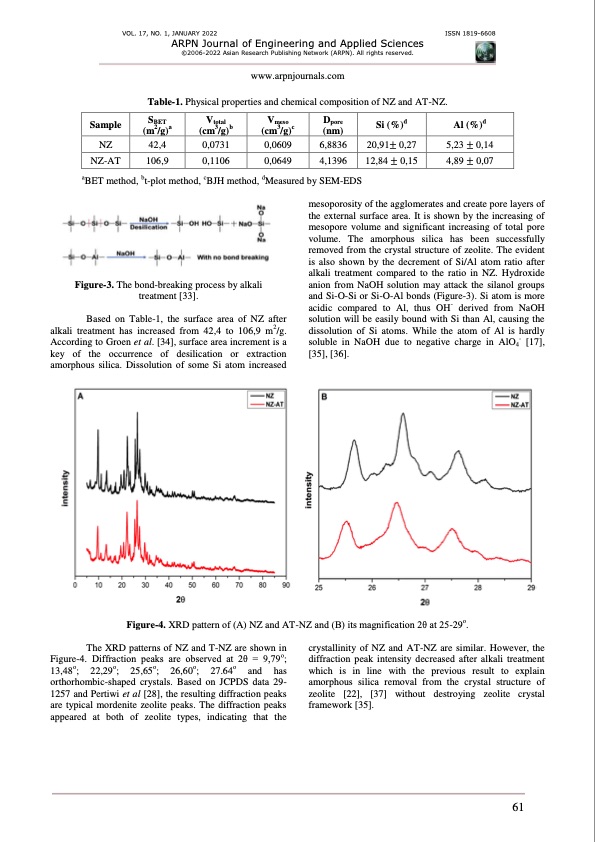
VOL. 17, NO. 1, JANUARY 2022 ISSN 1819-6608 ARPN Journal of Engineering and Applied Sciences ©2006-2022 Asian Research Publishing Network (ARPN). All rights reserved. www.arpnjournals.com NZ 42,4 NZ-AT 106,9 0,1106 0,0649 4,1396 12,84 ± 0,15 Table-1. Physical properties and chemical composition of NZ and AT-NZ. SVVDdd 0,0609 6,8836 20,91± 0,27 aBET method, bt-plot method, cBJH method, dMeasured by SEM-EDS 5,23 ± 0,14 4,89 ± 0,07 Sample BET (m2/g)a total (cm3/g)b meso pore Si (%) (cm3/g)c (nm) Al (%) 0,0731 Figure-3. The bond-breaking process by alkali treatment [33]. Based on Table-1, the surface area of NZ after alkali treatment has increased from 42,4 to 106,9 m2/g. According to Groen et al. [34], surface area increment is a key of the occurrence of desilication or extraction amorphous silica. Dissolution of some Si atom increased mesoporosity of the agglomerates and create pore layers of the external surface area. It is shown by the increasing of mesopore volume and significant increasing of total pore volume. The amorphous silica has been successfully removed from the crystal structure of zeolite. The evident is also shown by the decrement of Si/Al atom ratio after alkali treatment compared to the ratio in NZ. Hydroxide anion from NaOH solution may attack the silanol groups and Si-O-Si or Si-O-Al bonds (Figure-3). Si atom is more acidic compared to Al, thus OH- derived from NaOH solution will be easily bound with Si than Al, causing the dissolution of Si atoms. While the atom of Al is hardly soluble in NaOH due to negative charge in AlO - [17], [35], [36]. 4 Figure-4. XRD pattern of (A) NZ and AT-NZ and (B) its magnification 2θ at 25-29o. The XRD patterns of NZ and T-NZ are shown in Figure-4. Diffraction peaks are observed at 2θ = 9,79o; 13,48o; 22,29o; 25,65o; 26,60o; 27.64o and has orthorhombic-shaped crystals. Based on JCPDS data 29- 1257 and Pertiwi et al [28], the resulting diffraction peaks are typical mordenite zeolite peaks. The diffraction peaks appeared at both of zeolite types, indicating that the crystallinity of NZ and A T -NZ are similar. However, the diffraction peak intensity decreased after alkali treatment which is in line with the previous result to explain amorphous silica removal from the crystal structure of zeolite [22], [37] without destroying zeolite crystal framework [35]. 61PDF Image | ZEOLITE AND ACTIVATED CARBON FOR PROTON EXCHANGE MEMBRANE FUEL CELLS
PDF Search Title:
ZEOLITE AND ACTIVATED CARBON FOR PROTON EXCHANGE MEMBRANE FUEL CELLSOriginal File Name Searched:
jeas_0122_8819.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |