
PDF Publication Title:
Text from PDF Page: 006
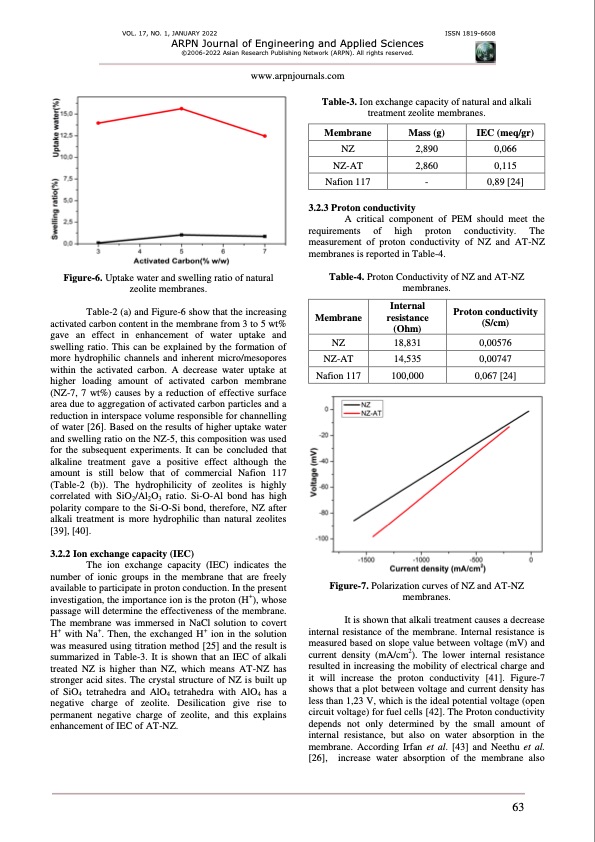
VOL. 17, NO. 1, JANUARY 2022 ISSN 1819-6608 ARPN Journal of Engineering and Applied Sciences ©2006-2022 Asian Research Publishing Network (ARPN). All rights reserved. www.arpnjournals.com Table-3. Ion exchange capacity of natural and alkali treatment zeolite membranes. Membrane NZ NZ-A T Nafion 117 Mass (g) 2,890 2,860 - IEC (meq/gr) 0,066 0,115 0,89 [24] Figure-6. Uptake water and swelling ratio of natural zeolite membranes. Table-2 (a) and Figure-6 show that the increasing activated carbon content in the membrane from 3 to 5 wt% gave an effect in enhancement of water uptake and swelling ratio. This can be explained by the formation of more hydrophilic channels and inherent micro/mesopores within the activated carbon. A decrease water uptake at higher loading amount of activated carbon membrane (NZ-7, 7 wt%) causes by a reduction of effective surface area due to aggregation of activated carbon particles and a reduction in interspace volume responsible for channelling of water [26]. Based on the results of higher uptake water and swelling ratio on the NZ-5, this composition was used for the subsequent experiments. It can be concluded that alkaline treatment gave a positive effect although the amount is still below that of commercial Nafion 117 (Table-2 (b)). The hydrophilicity of zeolites is highly correlated with SiO2/Al2O3 ratio. Si-O-Al bond has high polarity compare to the Si-O-Si bond, therefore, NZ after alkali treatment is more hydrophilic than natural zeolites [39], [40]. 3.2.2 Ion exchange capacity (IEC) The ion exchange capacity (IEC) indicates the number of ionic groups in the membrane that are freely available to participate in proton conduction. In the present investigation, the importance ion is the proton (H+), whose passage will determine the effectiveness of the membrane. The membrane was immersed in NaCl solution to covert H+ with Na+. Then, the exchanged H+ ion in the solution was measured using titration method [25] and the result is summarized in Table-3. It is shown that an IEC of alkali treated NZ is higher than NZ, which means AT-NZ has stronger acid sites. The crystal structure of NZ is built up of SiO4 tetrahedra and AlO4 tetrahedra with AlO4 has a negative charge of zeolite. Desilication give rise to permanent negative charge of zeolite, and this explains enhancement of IEC of AT-NZ. A critical component of PEM should meet the requirements of high proton conductivity. The measurement of proton conductivity of NZ and AT-NZ membranes is reported in Table-4. Table-4. Proton Conductivity of NZ and AT-NZ membranes. 3.2.3 Proton conductivity Membrane NZ NZ-A T Nafion 117 Internal resistance (Ohm) 18,831 14,535 100,000 Proton conductivity (S/cm) 0,00576 0,00747 0,067 [24] Figure-7. Polarization curves of NZ and AT-NZ membranes. It is shown that alkali treatment causes a decrease internal resistance of the membrane. Internal resistance is measured based on slope value between voltage (mV) and current density (mA/cm2). The lower internal resistance resulted in increasing the mobility of electrical charge and it will increase the proton conductivity [41]. Figure-7 shows that a plot between voltage and current density has less than 1,23 V, which is the ideal potential voltage (open circuit voltage) for fuel cells [42]. The Proton conductivity depends not only determined by the small amount of internal resistance, but also on water absorption in the membrane. According Irfan et al. [43] and Neethu et al. [26], increase water absorption of the membrane also 63PDF Image | ZEOLITE AND ACTIVATED CARBON FOR PROTON EXCHANGE MEMBRANE FUEL CELLS
PDF Search Title:
ZEOLITE AND ACTIVATED CARBON FOR PROTON EXCHANGE MEMBRANE FUEL CELLSOriginal File Name Searched:
jeas_0122_8819.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |