
PDF Publication Title:
Text from PDF Page: 004
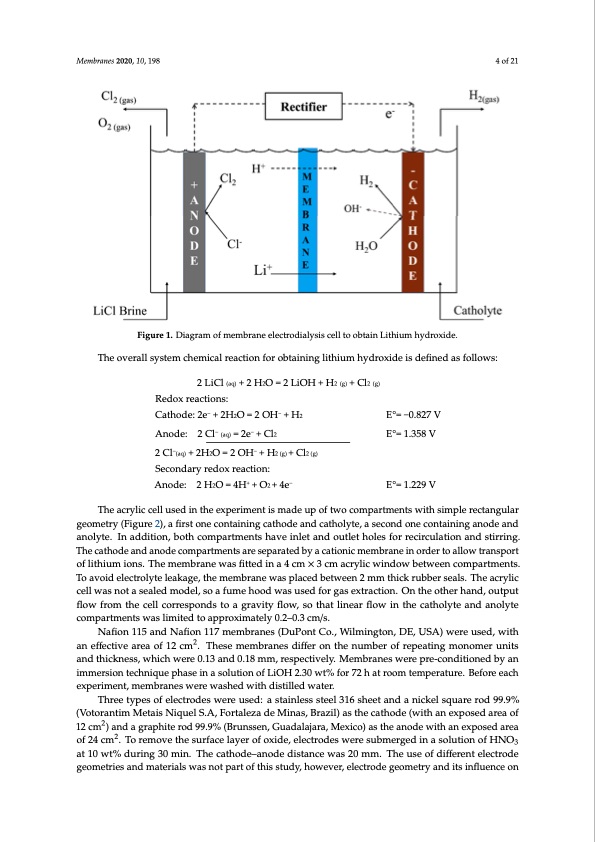
Membranes 2020, 10, x FOR PEER REVIEW 4 of 22 Membranes 2020, 10, x FOR PEER REVIEW Membranes 2020, 10, 198 4 of 22 4 of 21 128 129 Figure 1. DiagFraigmuoref m1.eDmiabgrraanme eolfecmtreomdibarlaynsieseclelclttrodoibatlayisnisLcitehlliutomohbytadinroLxidthei.um hydroxide. The overall system chemical reaction for obtaining lithium hydroxide is defined as follows: 130 The overall system chemical reaction for obtaining lithium hydroxide is defined as follows: Figure 1. Diagram of membrane electrodialysis cell to obtain Lithium hydroxide. 131 2 LiCl (aq) + 2 H2O = 2 LiOH + H2 (g) + Cl2 (g) 1T3h2e overall sRyestdeomx rcehaecmtiiocnasl:reaction for obtaining lithium hydroxide is defined as follows: 133 Cathode: 2e− + 2H2O = 2 OH− + H2 2LiCl(aq) +2H2O=2LiOH+H2 (g) +Cl2 (g) E°= −0.827 V E°= 1.358 V 1R3e4dox reactiAonso:de: 2 Cl− (aq) = 2e− + Cl2 Cathode:2e− +2H− 2O=2OH− +H2 − E°=−0.827V 135 2 Cl (aq) + 2H2O = 2 OH + H2 (g) + Cl2 (g) −− 1A3n6ode: 2 CSlec(aoq)n=d2aery+reCdl2ox reaction:E°= 1.358 V 137 − Anode: 2− H2O = 4H+ + O2 + 4e− E°= 1.229 V 138 The acrylic cell used in the experiment is made up of two compartments with simple rectangular 2 Cl (aq) + 2H2O = 2 OH + H2 (g) + Cl2 (g) Secondary redox reaction: The acrylic cell used in the experiment is made up of two compartments with simple rectangular 139 geometry (Figure 2), a first one containing cathode and catholyte, a second one containing anode and Anode: 2H2O=4H+ +O2+4e− E°=1.229V geometry (Figure 2), a first one containing cathode and catholyte, a second one containing anode and 140 anolyte. In addition, both compartments have inlet and outlet holes for recirculation and stirring. The The acrylic cell used in the experiment is made up of two compartments with simple rectangular anolyte. In addition, both compartments have inlet and outlet holes for recirculation and stirring. 141 cathode and anode compartments are separated by a cationic membrane in order to allow transport geometry (Figure 2), a first one containing cathode and catholyte, a second one containing anode and The cathode and anode compartments are separated by a cationic membrane in order to allow transport 142 of lithium ions. The membrane was fitted in a 4 cm × 3 cm acrylic window between compartments. anolyte. In addition, both compartments have inlet and outlet holes for recirculation and stirring. The of lithium ions. The membrane was fitted in a 4 cm × 3 cm acrylic window between compartments. 143 To avoid electrolyte leakage, the membrane was placed between 2 mm thick rubber seals. The acrylic cathode and anode compartments are separated by a cationic membrane in order to allow transport To avoid electrolyte leakage, the membrane was placed between 2 mm thick rubber seals. The acrylic 144 cell was not a sealed model, so a fume hood was used for gas extraction. On the other hand, output of lithium ions. The membrane was fitted in a 4 cm × 3 cm acrylic window between compartments. cell was not a sealed model, so a fume hood was used for gas extraction. On the other hand, output 145 flow from the cell corresponds to a gravity flow, so that linear flow in the catholyte and anolyte To avoid electrolyte leakage, the membrane was placed between 2 mm thick rubber seals. The acrylic flow from the cell corresponds to a gravity flow, so that linear flow in the catholyte and anolyte 146 compartments was limited to approximately 0.2–0.3 cm/s. cell was not a sealed model, so a fume hood was used for gas extraction. On the other hand, output compartments was limited to approximately 0.2–0.3 cm/s. flow from the cell corresponds to a gravity flow, so that linear flow in the catholyte and anolyte Nafion 115 and Nafion 117 membranes (DuPont Co., Wilmington, DE, USA) were used, with 2 Three types of electrodes were used: a stainless steel 316 sheet and a nickel square rod 99.9% (Votorantim Metais Niquel S.A, Fortaleza de Minas, Brazil) as the cathode (with an exposed area of 12 cm2) and a graphite rod 99.9% (Brunssen, Guadalajara, Mexico) as the anode with an exposed area of 24 cm2. To remove the surface layer of oxide, electrodes were submerged in a solution of HNO3 at 10 wt% during 30 min. The cathode–anode distance was 20 mm. The use of different electrode geometries and materials was not part of this study, however, electrode geometry and its influence on compartments was limited to approximately 0.2–0.3 cm/s. an effective area of 12 cm . These membranes differ on the number of repeating monomer units and thickness, which were 0.13 and 0.18 mm, respectively. Membranes were pre-conditioned by an immersion technique phase in a solution of LiOH 2.30 wt% for 72 h at room temperature. Before each experiment, membranes were washed with distilled water.PDF Image | Battery Grade Li Hydroxide by Membrane Electrodialysis
PDF Search Title:
Battery Grade Li Hydroxide by Membrane ElectrodialysisOriginal File Name Searched:
membranes-10-00198.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |