
PDF Publication Title:
Text from PDF Page: 005
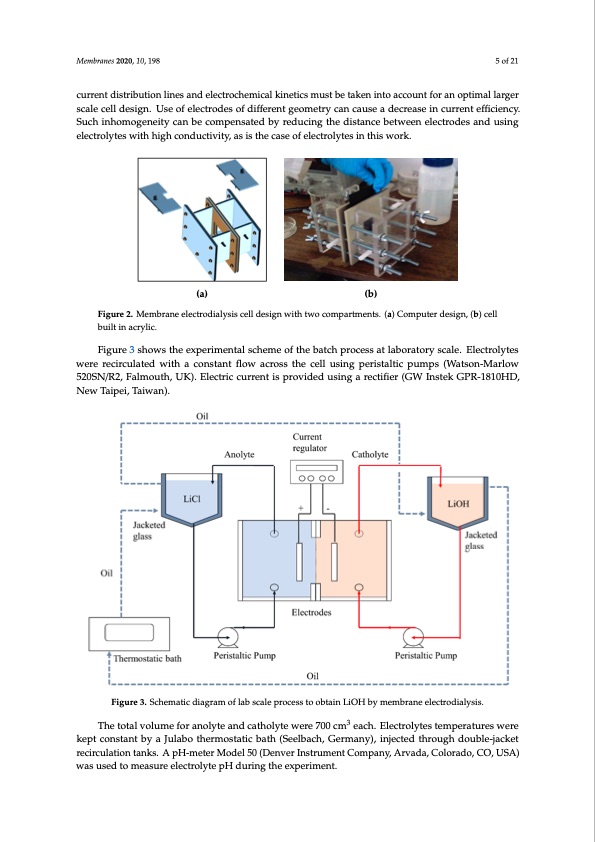
Membranes 2020, 10, 198 5 of 21 current distribution lines and electrochemical kinetics must be taken into account for an optimal larger scale cell design. Use of electrodes of different geometry can cause a decrease in current efficiency. Such inhomogeneity can be compensated by reducing the distance between electrodes and using Membranes 2020, 10, x FOR PEER REVIEW 5 of 22 electrolytes with high conductivity, as is the case of electrolytes in this work. (a) (b) Figure 2. Membrane electrodialysis cell design with two compartments. (a) Computer design, (b) cell Figure 2. Membrane electrodialysis cell design with two compartments. (a) Computer design, (b) cell built in acrylic. built in acrylic. Figure 3 shows the experimental scheme of the batch process at laboratory scale. Electrolytes Nafion 115 and Nafion 117 membranes (DuPont Co., Wilmington, DE, USA) were used, with an were recirculated with a constant flow across the cell using peristaltic pumps (Watson-Marlow effective area of 12 cm2. These membranes differ on the number of repeating monomer units and 520SN/R2, Falmouth, UK). Electric current is provided using a rectifier (GW Instek GPR-1810HD, thickness, which were 0.13 and 0.18 mm, respectively. Membranes were pre-conditioned by an NMewemTbrainpesei2,0T20a,i1w0,axnF)O. R PEER REVIEW 6 of 22 immersion technique phase in a solution of LiOH 2.30 wt% for 72 h at room temperature. Before each experiment, membranes were washed with distilled water. Three types of electrodes were used: a stainless steel 316 sheet and a nickel square rod 99.9% (Votorantim Metais Niquel S.A, Fortaleza de Minas, Brazil) as the cathode (with an exposed area of 12 cm2) and a graphite rod 99.9% (Brunssen, Guadalajara, Mexico) as the anode with an exposed area of 24 cm2. To remove the surface layer of oxide, electrodes were submerged in a solution of HNO3 at 10 wt% during 30 min. The cathode–anode distance was 20 mm. The use of different electrode geometries and materials was not part of this study, however, electrode geometry and its influence on current distribution lines and electrochemical kinetics must be taken into account for an optimal larger scale cell design. Use of electrodes of different geometry can cause a decrease in current efficiency. Such inhomogeneity can be compensated by reducing the distance between electrodes and using electrolytes with high conductivity, as is the case of electrolytes in this work. Figure 3 shows the experimental scheme of the batch process at laboratory scale. Electrolytes were recirculated with a constant flow across the cell using peristaltic pumps (Watson-Marlow 520SN/R2, Falmouth, UK). Electric current is provided using a rectifier (GW Instek GPR-1810HD, New Taipei, Taiwan). The total volume for anolyte and catholyte were 700 cm3 each. Electrolytes temperatures were kept constant by a Julabo thermostatic bath (Seelbach, Germany), injected through double-jacket recirculation tanks. A pH-meter Model 50 (Denver Instrument Company, Arvada, Colorado, CO, USA) was used to measure electrolyte pH during the experiment. Figure 3. Schematic diagram of lab scale process to obtain LiOH by membrane electrodialysis. Figure 3. Schematic diagram of lab scale process to obtain LiOH by membrane electrodialysis. The total volume for anolyte and catholyte were 700 cm3 each. Electrolytes temperatures were Theoretical mass transfer of lithium ions (mtheor ) through the membrane is determined by kept constant by a Julabo thermostatic bath (Seelbach, Germany), injected through double-jacket Faraday's laws of electrolysis (Equation (1)): recirculation tanks. A pH-meter Model 50 (Denver Instrument Company, Arvada, Colorado, CO, USA) was used to measure electrolyte pH during the experiment. mtheor= z·F (1) Where I is current intensity (A), M is molecular weight (g/mol), z is the number of electrons per ion, F is Faraday's constant (96484.5 As/mol) and t is the time interval (s). The current efficiency (∅) was calculated by equation (2): I·t·M z·FPDF Image | Battery Grade Li Hydroxide by Membrane Electrodialysis
PDF Search Title:
Battery Grade Li Hydroxide by Membrane ElectrodialysisOriginal File Name Searched:
membranes-10-00198.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |