
PDF Publication Title:
Text from PDF Page: 005
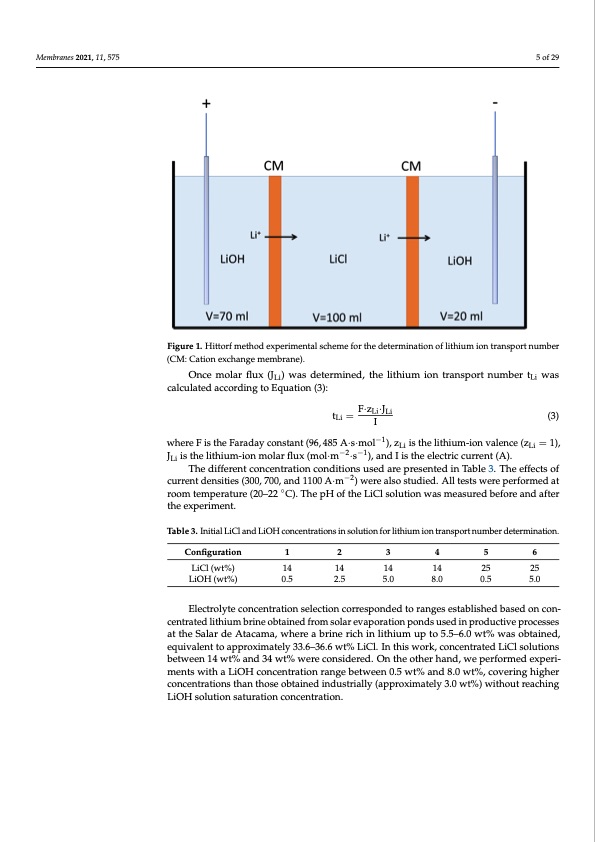
−1 i d o o i o S 4 o Membranes 2021, 11, 575 5 of 29 where F is the Faraday constant (96,485 A ∙ s ∙ mol ), zLi is the lithium-ion valence (zL 1), JLi is the lithium-ion molar flux (mol ∙ m−2 ∙ s−1), and I is the electric current (A). The different concentration conditions used are presented in Table 3. The effects current densities (300, 700, and 1100 A∙m−2) were also studied. All tests were performe room temperature (20–22°C). The pH of the LiCl solution was measured before and af the experiment. Figure 1. Hittorf method experimental scheme for the determination of lithium ion transport number (CM: Cation ex- Figure 1. Hittorf method experimental scheme for the determination of lithium ion transport number change membrane). (CM: Cation exchange membrane). Once molar flux (JLi) was determined, the lithium ion transport number tLi was Table 3. Initial LiCl and LiOH concentrations in solution for lithium ion transport number determination. calculated according to Equation (3): Configuration LiCl (wt%) LiOH (wt%) 1 2 2.5 3 4 14 14 5.0 8.0 5 6 25 25 14 F·zLi·JLi14 tLi = I 0.5 (3) where F is the Faraday constant (96, 485 A·s·mol−1), zLi is the lithium-ion valence (zLi = 1), Electrolyte concentratio−n2se−le1ction corresponded to ranges established based on c JLi is the lithium-ion molar flux (mol·m ·s ), and I is the electric current (A). centrated lithium brine obtained from solar evaporation ponds used in productive p The different concentration conditions used are presented in Table 3. The effects of cesses at the Salar de Atacama, where a brine rich in lithium up to 5.5–6.0 wt% was current densities (300, 700, and 1100 A·m−2) were also studied. All tests were performed at the experiment. ◦ tained, equivalent to approximately 33.6–36.6 wt% LiCl. In this work, concentrated L room temperature (20–22 C). The pH of the LiCl solution was measured before and after solutions between 14 wt% and 34 wt% were considered. On the other hand, we perform experiments with a LiOH concentration range between 0.5 wt% and 8.0 wt%, coveri Table3.InitihaligLhiCelrancodnLciOenHtrcaotnicoenstrathtioanstihnososleutoiobntafoinrelidthiunmduiosntrtiralnlsypo(artpnpurmobxeimrdaeterlymi3n.a0tiwont.%)with reaching LiOH solution saturation concentration. Configuration 1 2 3 4 5 6 0.5 5.0 LiCl(w2.t4%.)LinearSweep14Voltammetr1y4(LSV) 14 14 25 25 LiOH (wt%) 0.5 2.5 5.0 8.0 0.5 5.0 In the characterization of bipolar membranes, the linear sweep voltammetry (L technique allows the determination of the degree of salt leakage that occurs through the Electroalnydteocfothnecelnimtriattaitoionnsseloefcwtioantecrodrirfefuspsiondteodwtaordrasnbgiepsoelasrtambleimshberdanbeasredacotinvecoint-erface [ centratedliItnhiuthmebcraisneofbtcaaintieodn-feroxmchsaonlgaeremvaepmobraratinoensp,oLnSdVscuasnedbienupsroedutcotidvetperomceinsseetshelimiti at the SalarcudrereAntadceanmsait,ywcahuesredabyritnhe rpiochlarinizalitihoinucmonucpentotra5t.i5o–n6.e0ffwectt%atwmaesmobrtainneesdu,rface. B equivalent ptoheanpopmroexnima atfefelyct3t3h.6e–e3f6fi.c6iewntc%y LofiCthl.eInelethcitsrowdoiarlky,sciosnpcreonctersast.ed LiCl solutions between 14 wt% and 34 wt% were considered. On the other hand, we performed experi- ments with a LiOH concentration range between 0.5 wt% and 8.0 wt%, covering higher concentrations than those obtained industrially (approximately 3.0 wt%) without reaching LiOH solution saturation concentration.PDF Image | Bipolar Membrane Electrodialysis for LiOH Production
PDF Search Title:
Bipolar Membrane Electrodialysis for LiOH ProductionOriginal File Name Searched:
membranes-11-00575-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |