
PDF Publication Title:
Text from PDF Page: 006
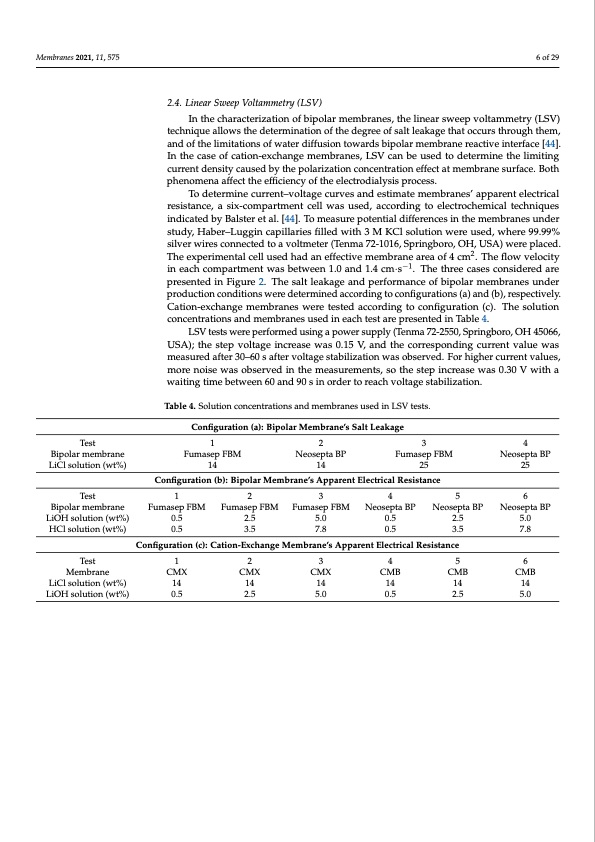
Membranes 2021, 11, 575 6 of 29 2.4. Linear Sweep Voltammetry (LSV) In the characterization of bipolar membranes, the linear sweep voltammetry (LSV) technique allows the determination of the degree of salt leakage that occurs through them, and of the limitations of water diffusion towards bipolar membrane reactive interface [44]. In the case of cation-exchange membranes, LSV can be used to determine the limiting current density caused by the polarization concentration effect at membrane surface. Both phenomena affect the efficiency of the electrodialysis process. To determine current–voltage curves and estimate membranes’ apparent electrical resistance, a six-compartment cell was used, according to electrochemical techniques indicated by Balster et al. [44]. To measure potential differences in the membranes under study, Haber–Luggin capillaries filled with 3 M KCl solution were used, where 99.99% silver wires connected to a voltmeter (Tenma 72-1016, Springboro, OH, USA) were placed. The experimental cell used had an effective membrane area of 4 cm2. The flow velocity in each compartment was between 1.0 and 1.4 cm·s−1. The three cases considered are presented in Figure 2. The salt leakage and performance of bipolar membranes under production conditions were determined according to configurations (a) and (b), respectively. Cation-exchange membranes were tested according to configuration (c). The solution concentrations and membranes used in each test are presented in Table 4. LSV tests were performed using a power supply (Tenma 72-2550, Springboro, OH 45066, USA); the step voltage increase was 0.15 V, and the corresponding current value was measured after 30–60 s after voltage stabilization was observed. For higher current values, more noise was observed in the measurements, so the step increase was 0.30 V with a waiting time between 60 and 90 s in order to reach voltage stabilization. Table 4. Solution concentrations and membranes used in LSV tests. Configuration (a): Bipolar Membrane’s Salt Leakage Test 1 2 3 4 Bipolar membrane Fumasep FBM Neosepta BP Fumasep FBM Neosepta BP LiCl solution (wt%) 14 14 25 25 Configuration (b): Bipolar Membrane’s Apparent Electrical Resistance Test 1 2 3 4 5 6 Bipolar membrane LiOH solution (wt%) HCl solution (wt%) Fumasep FBM 0.5 0.5 Fumasep FBM 2.5 3.5 Fumasep FBM 5.0 7.8 Neosepta BP 0.5 0.5 Neosepta BP 2.5 3.5 Neosepta BP 5.0 7.8 Configuration (c): Cation-Exchange Membrane’s Apparent Electrical Resistance Test 1 2 3 4 5 6 Membrane LiCl solution (wt%) LiOH solution (wt%) CMX CMX 14 14 0.5 2.5 CMX CMB 14 14 5.0 0.5 CMB CMB 14 14 2.5 5.0PDF Image | Bipolar Membrane Electrodialysis for LiOH Production
PDF Search Title:
Bipolar Membrane Electrodialysis for LiOH ProductionOriginal File Name Searched:
membranes-11-00575-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |