
PDF Publication Title:
Text from PDF Page: 011
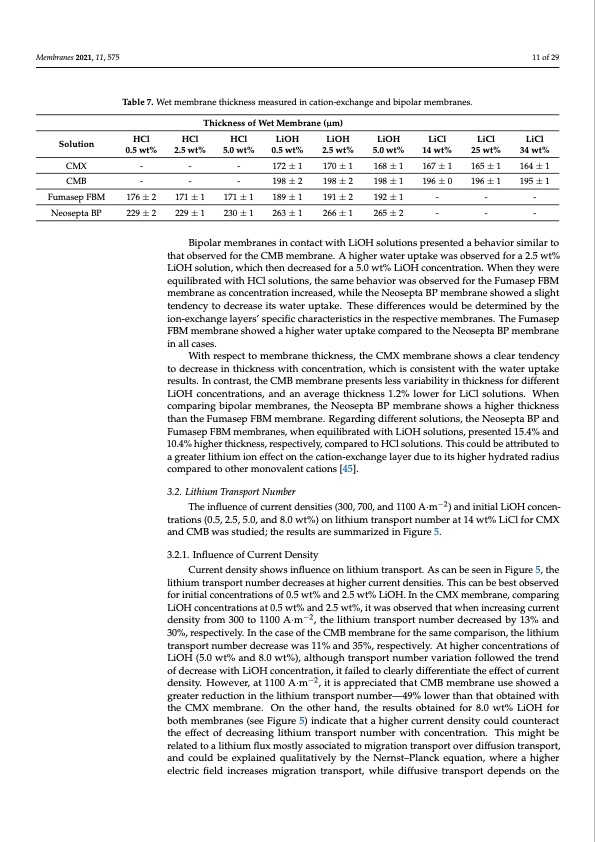
Membranes 2021, 11, 575 11 of 29 Table 7. Wet membrane thickness measured in cation-exchange and bipolar membranes. Thickness of Wet Membrane (μm) Solution HCl HCl HCl LiOH 0.5 wt% 2.5 wt% 5.0 wt% 0.5 wt% LiOH 2.5 wt% 170±1 198±2 191±2 266±1 LiOH LiCl LiCl 5.0 wt% 14 wt% 25 wt% LiCl 34 wt% CMX---172±1 CMB---198±2 FumasepFBM 176±2 171±1 171±1 189±1 NeoseptaBP 229±2 229±1 230±1 263±1 168±1 167±1 165±1 164±1 198±1 196±0 196±1 195±1 192±1--- 265±2--- Bipolar membranes in contact with LiOH solutions presented a behavior similar to that observed for the CMB membrane. A higher water uptake was observed for a 2.5 wt% LiOH solution, which then decreased for a 5.0 wt% LiOH concentration. When they were equilibrated with HCl solutions, the same behavior was observed for the Fumasep FBM membrane as concentration increased, while the Neosepta BP membrane showed a slight tendency to decrease its water uptake. These differences would be determined by the ion-exchange layers’ specific characteristics in the respective membranes. The Fumasep FBM membrane showed a higher water uptake compared to the Neosepta BP membrane in all cases. With respect to membrane thickness, the CMX membrane shows a clear tendency to decrease in thickness with concentration, which is consistent with the water uptake results. In contrast, the CMB membrane presents less variability in thickness for different LiOH concentrations, and an average thickness 1.2% lower for LiCl solutions. When comparing bipolar membranes, the Neosepta BP membrane shows a higher thickness than the Fumasep FBM membrane. Regarding different solutions, the Neosepta BP and Fumasep FBM membranes, when equilibrated with LiOH solutions, presented 15.4% and 10.4% higher thickness, respectively, compared to HCl solutions. This could be attributed to a greater lithium ion effect on the cation-exchange layer due to its higher hydrated radius compared to other monovalent cations [45]. 3.2. Lithium Transport Number The influence of current densities (300, 700, and 1100 A·m−2) and initial LiOH concen- trations (0.5, 2.5, 5.0, and 8.0 wt%) on lithium transport number at 14 wt% LiCl for CMX and CMB was studied; the results are summarized in Figure 5. 3.2.1. Influence of Current Density Current density shows influence on lithium transport. As can be seen in Figure 5, the lithium transport number decreases at higher current densities. This can be best observed for initial concentrations of 0.5 wt% and 2.5 wt% LiOH. In the CMX membrane, comparing LiOH concentrations at 0.5 wt% and 2.5 wt%, it was observed that when increasing current density from 300 to 1100 A·m−2, the lithium transport number decreased by 13% and 30%, respectively. In the case of the CMB membrane for the same comparison, the lithium transport number decrease was 11% and 35%, respectively. At higher concentrations of LiOH (5.0 wt% and 8.0 wt%), although transport number variation followed the trend of decrease with LiOH concentration, it failed to clearly differentiate the effect of current density. However, at 1100 A·m−2, it is appreciated that CMB membrane use showed a greater reduction in the lithium transport number—49% lower than that obtained with the CMX membrane. On the other hand, the results obtained for 8.0 wt% LiOH for both membranes (see Figure 5) indicate that a higher current density could counteract the effect of decreasing lithium transport number with concentration. This might be related to a lithium flux mostly associated to migration transport over diffusion transport, and could be explained qualitatively by the Nernst–Planck equation, where a higher electric field increases migration transport, while diffusive transport depends on thePDF Image | Bipolar Membrane Electrodialysis for LiOH Production
PDF Search Title:
Bipolar Membrane Electrodialysis for LiOH ProductionOriginal File Name Searched:
membranes-11-00575-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |