
PDF Publication Title:
Text from PDF Page: 009
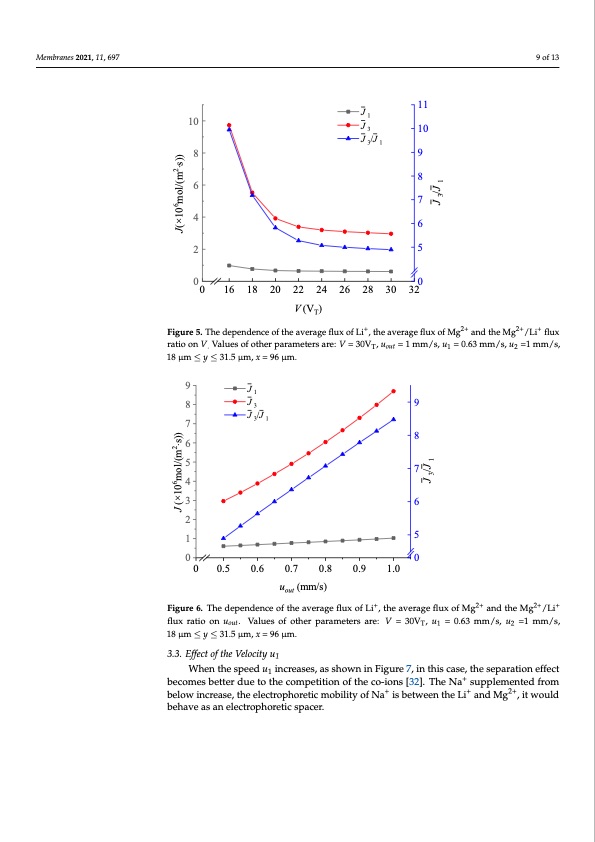
Membranes 2021, 11, x FOR PEER REVIEW 9 of 13 J 11 10 1 Membranes 2021, 11, 697 J 3 10 9 of 13 Membranes 2021, 11, x FOR PEER REVIEW 8 10 8 J3 10 J 3/J 1 6J1 9 9of13 11 7 4 J 3/J 1 8 8 69 2 5 7 6 00 40 16 18 20 22 24 26 28 30 326 2 V (VT) 5 Figure 5. The dependence of the average flux of Li+, the average flux of Mg2+ and the Mg2+/Li+ flux ratio on V. Values of other parameters are: V = 30VT, uout = 1 mm/s, u1 = 0.63 mm/s, u2 =1 mm/s, 18 μm ≤ y ≤ 301.5 μm, x = 96 μm. 0 0 16 18 20 22 24 26 28 30 32 3.2. Effect of the Velocity uout V (VT) As shown in Figure 6, when the horizontal speed uout increases, the Mg2+/Li+ flux ratio Figure 5. The dependence of the average flux of+Li+, the average flux of M2+g2+ and the M2+g2+/L+i+ flux Figure 5. The dependence of the average flux of Li , the average flux of Mg and the Mg /Li flux increased. Although the flux of Li+ ions increases, there is not enough time for Mg2+ to ratio on V. Values of other parameters are: V = 30VT, uout = 1 mm/s, u1 = 0.63 mm/s, u2 =1 mm/s, 18 μm ratio on V Values of other parameters are: V = 30V , u = 1 mm/s, u = 0.63 mm/s, u =1 mm/s, . Tout 1 22+ reach the downside reservoir, resulting in a significant rise in the average flux of Mg and ≤ y ≤ 31.5 μm, x = 96 μm. 18 μm ≤ y ≤ 31.5 μm, x = 96 μm. poor separation effect, specifically reflected in the increase in the Mg2+/Li+ flux ratio. 3.2. Effect of the Velocity uout 9 incr8eased. Althoug3h the flux of Li ions increases, there is not enough time for Mg to J 2+ + As shown in F1igure 6, when the horizontal speed uout increases, the Mg /Li flux ratio J + 9 2+ J /J 2+ reach the downside3 re1servoir, resulting in a significant rise in the average flux of Mg and 7 poor separation effect, specifically reflected in the increase in the Mg2+/Li+ flux ratio. 68 9 J1 8J39 5 4 7 6 8 J 3/J 1 6 7 3 2 5 4 5 17 00 30 0.5 0.6 0.7 0.8 0.9 1.0 2 uout (mm/s) Fiigur1ree66..ThedependenceoftheaveragefflluxofLi,,ththeeaavveerraaggeeflfluuxxooffMgg anadndthtehMeMgg/Li/fLluix ratio on uout. Values of other parameters are: V = 30VT, u1 = 0.63 mm/s, u2 =1 mm/s, 18 μm ≤ y ≤ 31.5 flux ratio on uout. Values of other parameters are: V = 30VT, u1 = 0.63 mm/s, u2 =1 mm/s, 00 μm, x = 96 μm. 18 μm ≤ y ≤ 31.5 μm, x = 96 μm. 0 0.5 0.6 0.7 0.8 0.9 1.0 3..3.. Efffectt off tthee Veelloocciittyu1 1 uout (mm/s) Figure 6. The dependence of the average flux of Li+, the average flux of Mg2+ and the Mg2+/Li+ flux + becomes bettterrdueettooththeeccoomppeetittiitoionnooffththeecoco-i-oionnss[3[23]2.].TThheeNNaa+ssuuppplelemeenntteedfrfroom ratio on uout. Values of other parameters are: V = 30VT, u1 = 0.63 mm/s, u2 =1 mm/s, 18 μm ≤ y ≤ 31.5 + +2+ below increase, the electrophoretic mobility of Na +is between the Li +and Mg 2+, it would below increase, the electrophoretic mobility of Na is between the Li and Mg , it would μm, x = 96 μm. behave as an electrophoretic spacer. behave as an electrophoretic spacer. 3.3. Effect of the Velocity u1 When the speed u1 increases, as shown in Figure 7, in this case, the separation effect becomes better due to the competition of the co-ions [32]. The Na+ supplemented from below increase, the electrophoretic mobility of Na+ is between the Li+ and Mg2+, it would behave as an electrophoretic spacer. 6 + 5 2+2+ 2+ 2++ + Whenttheesspeeedu1 iincreases,,asshowniinFiigurree77,,iintthiissccaassee,,tthheesseeppaarraattioionneefffeecctt 1 J (×106mol/(m2⋅sJ))(×106mol/(m2⋅s)) J(×106mol/(m2⋅sJ)()×106mol/(m2⋅s)) J 3/J 1 J 3/J 1 J 3/J 1 J 3/J 1PDF Image | Brines Based on Free Flow Ion Concentration Polarization
PDF Search Title:
Brines Based on Free Flow Ion Concentration PolarizationOriginal File Name Searched:
membranes-11-00697-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |