
PDF Publication Title:
Text from PDF Page: 010
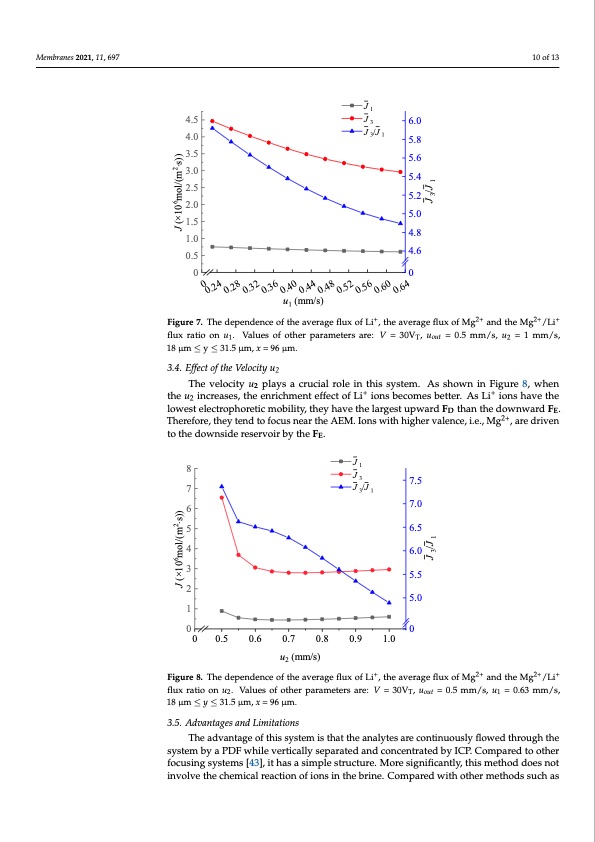
Membranes 2021, 11, 697 10 of 13 Membranes 2021, 11, x FOR PEER REVIEW Membranes 2021, 11, x FOR PEER REVIEW J3/J1 4.0 31 10 of 13 2.0 1.5 1.5 1.0 1.0 J J1 10 of 13 4.5 J 1 4.5 J3 6.0 J 3/J 6.0 4.0 5.8 5.8 3.5 3.5 3.0 3.0 2.5 2.5 2.0 5.6 5.6 5.4 5.4 5.2 5.2 5.0 5.0 4.8 4.8 4.6 4.6 00 00 u (mm/s) u1 (mm/s) 0.5 0.5 3.4. Effect of the Velocity u2 1 2+2+ 2+ 2+ + + Figure 7. The dependence of the average flux of Li , the average flux of Mg and the Mg /Li flux ratio on u1. Values of other parameters are: V = 30VT, uout = 0.5 mm/s, u2 = 1 mm/s, 18 μm ≤ y ≤ 31.5 flruaxtioraotniouo1.nVualu.esVoafluoetsheorfpoatrhaemreptearsamaret:eVrs=a3r0eV:TV,uo=ut =300V.5,mum/s,=u20=.51mm/s,1u8μ=m1≤mym≤3/1s.,5 + FFigiguurree77..ThedependenceoftheaverageflfluxofLi+,,ttheeaaveerraaggeefflluuxxooffMgg2+ anadndthteheMMg2g+/Li/+Lfliux μm, x = 96 μm.1 1μ8mμm, x≤= 9y6≤μm31..5 μm, x = 96 μm. T out 2 33.4.4. .EfffeeccttoofftthheeVeeloloccitityyuu2 2 TThheevellocity u2 pllaayyssaacrcurucicailarlorloeleininthitshsisystyesmte.mA.s sAhsowshnoiwnnFiignurFeig8u,rweh8e,nwthenu2 2 The velocity u2 plays a crucial role in this system. As shown in Figure 8, when the u2 ++ ++ tihnecrueaisnecs,retahseese,ntrhicehemnerincthemffenct eoffeLcit oiof nLsi bieocnosmbeescboemttesr.bAetsteLr.i AiosnLsi haiovnestheavloewtheest 2++ increases, the enrichment effect of Li ions becomes better. As Li ions have the lowest lowest electrophoretic mobility, they have the largest upward F than the downward F . electrophoretic mobility, they have the largest upward FD thanDthe downward FE. TherEe- electrophoretic mobility, they have the largest upward FD than the downward FE. There- Therefore, they tend to focus near the AEM. Ions with higher valence, i.e., M2g+ 2+, are driven fore, they tend to focus near the AEM. Ions with higher valence, i.e., Mg2+, are driven to fore, they tend to focus near the AEM. Ions with higher valence, i.e., Mg , are driven to to the downside reservoir by the F . the downside reservoir by the FE. E the downside reservoir by the FE. 8 J1 8 J 1 J J /J 7 J3/J1 731 7.5 6 5 5 4 4 6.0 6.0 5.5 5.5 5.0 5.0 u (mm/s) u2 (mm/s) J3 3 7.5 6 7.0 7.0 6.5 6.5 3 3 2 2 1 1 00 00 0.5 0.6 0.7 0.8 0.9 1.0 0 0 0.5 0.6 0.7 0.8 0.9 1.0 μm, x = 96 μm. 18 μm ≤ y ≤ 31.5 μm, x = 96 μm. 2 + 2+2++ Figure 8. The dependence of the average flux of Li+, the average flux of Mg2+2+and the Mg2+/2L+i+flu+x FFigiguurere88..TThheedeepeendenceoffttheaverageflfluxofLi ,,ttheaverrageefflluxoffMgg anadndthteheMMgg/Li/Lfliux ratio on u2. Values of other parameters are: V = 30VT, uout = 0.5 mm/s, u1 = 0.63 mm/s, 18 μm ≤ y ≤ 31.5 ratio on u2. Values of other parameters are: V = 30VT, uout = 0.5 mm/s, u1 = 0.63 mm/s, 18 μm ≤ y ≤ 31.5 flux ratio on u2. Values of other parameters are: V = 30VT, uout = 0.5 mm/s, u1 = 0.63 mm/s, μm, x = 96 μm. 33.5.5. .AddvvaanttaaggeessaannddLLimimititaattioionnss 3.5. Advantages and Limitations TThheeaaddvvaanntataggeeofotfhtihsisysytesmtemis tishatthtahtethaenalnyatelystaerseacroentcionnutoinuusloyuflsloywfelodwtherdouthgrhotuhgeh The advantage of this system is that the analytes are continuously flowed through styhsetesmysbteyma bPyDFa wPDhiFlewvheirlteicvaellrytisceapllayrasetepdaraantdedcoancdencotrnactendtrbayteIdCPb.yCIoCmPp. aCroedmtpoaorethdetro the system by a PDF while vertically separated and concentrated by ICP. Compared to fotchuesrinfogcsuyssitnegmssys[4te3m], sit[4h3a]s, ait shiamspalseimstrpulectsutreu.cMtuorer.eMsiogrneifisicganitfliyc,atnhtilsy,mtheitshmodetdhoedsdnoets other focusing systems [43], it has a simple structure. More significantly, this method does invootlivnevothlveecthemchiceamlriecacltrieoanctoiofnioonfsiionntshienbthrienbe.riCnoe.mCpoamrepdarweidthwoitheorthmeertmhoedthsosduschsuacsh not involve the chemical reaction of ions in the brine. Compared with other methods such + as nanofiltration membrane [14]. Li et al. increased the Li+ yield and improved the sepa- as nanofiltration membrane [14]. Li et al. increased the Li yield and improved the sepa- 2+ + 2+ ration effect of Mg2+ and Li+ by increasing the operating pressure. The Mg2+ rejection rate ration effect of Mg and Li by increasing the operating pressure. The Mg rejection rate 66 22 JJ ( (× ×1100 m mool l/ /( (m m ⋅ s⋅ s) ) ) ) 66 22 JJ ( (× ×1100 m mool l/ /( (m m ⋅ s⋅ s) ) ) ) JJ //JJ 33 11 JJ //JJ 33 11 0 0 0.24 0.240.28 0.280.32 0.320.36 0.360.40 0.400.44 0.440.48 0.480.52 0.520.56 0.560.60 0.600.64 0.64PDF Image | Brines Based on Free Flow Ion Concentration Polarization
PDF Search Title:
Brines Based on Free Flow Ion Concentration PolarizationOriginal File Name Searched:
membranes-11-00697-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |