
PDF Publication Title:
Text from PDF Page: 003
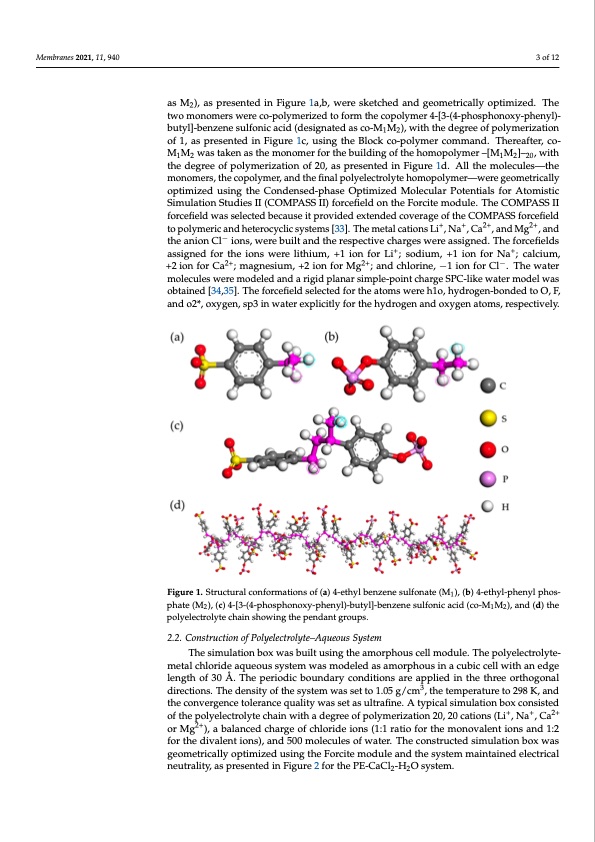
Membranes 2021, 11, 940 The structural backbone of typical cation-exchange membranes generally contains fixed-charge acid groups, mainly the sulfonic, the phosphoric, or the carboxylic acid groups. The present membrane material was constructed by mixing two aromatic moie- ties containing the sulfonic and the phosphoric groups. Initially, the two monomers, ignated as M2), as presented in Figure 1a,b, were sketched and geometrically optimized. The two monomers were co-polymerized to form the copolymer 4-[3-(4-phosphonoxy- pahsenMyl),-baustpyrle]-sbeenntezdenien Fsiugluforeni1ca,bac,iwder(edesskiegtnchaeteddanads gceoo-mMe1Mtri2c)a,llwy iotphtimthiezed.egTrhee of 2 ptowlyomeornizoamtieornswoefr1e,caos-pporlyemseenrtiezdedintoFfoigrmurtehe1c,ouposilnymgetrh4e-[B3l-o(4c-kphcos-phoolynmoxeyr-pchoemnmyl)a-nd. Tbhuetryela]-fbtern,zceon-eMsu1Mlfo2nwicaasctiadk(ednesaigsntahtedmaosncom-Me1rMfo2r),twhiethbtuhielddinegreoefothfpeohlyomeorpizoaltyimoner – of 1, as presented in Figure 1c, using the Block co-polymer command. Thereafter, co- [M1M2]–20, with the degree of polymerization of 20, as presented in Figure 1d. All the mol- M M was taken as the monomer for the building of the homopolymer –[M M ]– , with ecu1les2—the monomers, the copolymer, and the final polyelectrolyte homo1po2lym20er—were the degree of polymerization of 20, as presented in Figure 1d. All the molecules—the geometrically optimized using the Condensed-phase Optimized Molecular Potentials for namely, 4-ethylbenzene sulfonate (designated as M1) and 4-ethyl-phenyl phosphate (des- COMPASS II forcefield was selected because it provided extended coverage of the COM- Simulation Studies II (COMPASS II) forcefield on the Forcite module. The COMPASS II PASS forcefield to polymeric and heterocyclic systems [33]. The metal cations Li+, Na+, forcefield was selected because it provided extended coverage of the COMPASS forcefield Ca2+, and Mg2+, and the anion Cl- ions, were built and the respective charges were assigned. to polymeric and heterocyclic systems [33]. The metal cations Li+, Na+, Ca2+, and Mg2+, and The forcefield−s assigned for the ions were lithium, +1 ion for Li+; sodium, +1 ion for Na+; the anion Cl ions, were built and the respective charges were assigned. The forcefields 3 of 12 monomers, the copolymer, and the final polyelectrolyte homopolymer—were geometrically Atomistic Simulation Studies II (COMPASS II) forcefield on the Forcite module. The optimized using the Condensed-phase Optimized Molecular Potentials for Atomistic 2+ 2+ - calcium,+2ionforCa ;magnesium,+2ionforMg+;andchlorine,-1ionfor+Cl.Thewater assigned for the ions were lithium, +1 ion for Li ; sodium, +1 ion for Na ; calcium, molecules we2r+e modeled and a rigid plana2+r simple-point charge SPC-li−ke water model +2 ion for Ca ; magnesium, +2 ion for Mg ; and chlorine, −1 ion for Cl . The water was obtained [34,35]. The forcefield selected for the atoms were h1o, hydrogen-bonded to molecules were modeled and a rigid planar simple-point charge SPC-like water model was Oo,bFta,iannedo[324*,3o5x].yTgheen,fosrpc3efiinelwdsaetelercetexdplfiocritlhyefaotrotmhsewhyerderohg1oe,nhaynddroogxeyng-beonnadteodmtso,Ore,sFp,ec- tiavnedlyo.2*, oxygen, sp3 in water explicitly for the hydrogen and oxygen atoms, respectively. FFigiguurree11..Sttrructural conformationsoff(a(a))44-e-tehthyyl blebneznezneenseuslufolnfoanteat(eM(M),1()b,)(b4-)e4th-eytlh-pyhl-epnhyelnpyhlopsh-os- 1 phate (M2), (c) 4-[3-(4-phosphonoxy-phenyl)-butyl]-benzene sulfonic acid (co-M1M2), and (d) the phate (M2), (c) 4-[3-(4-phosphonoxy-phenyl)-butyl]-benzene sulfonic acid (co-M1M2), and (d) the polyelectrolyte chain showing the pendant groups. polyelectrolyte chain showing the pendant groups. 2.2. Construction of Polyelectrolyte–Aqueous System 2.2. Construction of Polyelectrolyte–Aqueous System The simulation box was built using the amorphous cell module. The polyelectrolyte- The simulation box was built using the amorphous cell module. The polyelectrolyte- metal chloride aqueous system was modeled as amorphous in a cubic cell with an edge metal chloride aqueous system was modeled as amorphous in a cubic cell with an edge length of 30 Å. The periodic boundary conditions are applied in the three orthogonal directions. The density of the system was set to 1.05 g/cm3, the temperature to 298 K, and the convergence tolerance quality was set as ultrafine. A typical simulation box consisted of the polyelectrolyte chain with a degree of polymerization 20, 20 cations (Li+, Na+, Ca2+ or Mg2+), a balanced charge of chloride ions (1:1 ratio for the monovalent ions and 1:2 for the divalent ions), and 500 molecules of water. The constructed simulation box was geometrically optimized using the Forcite module and the system maintained electrical neutrality, as presented in Figure 2 for the PE-CaCl2-H2O system.PDF Image | Diffusion of Monovalent Ions in Polyelectrolyte
PDF Search Title:
Diffusion of Monovalent Ions in PolyelectrolyteOriginal File Name Searched:
membranes-11-00940-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |