
PDF Publication Title:
Text from PDF Page: 005
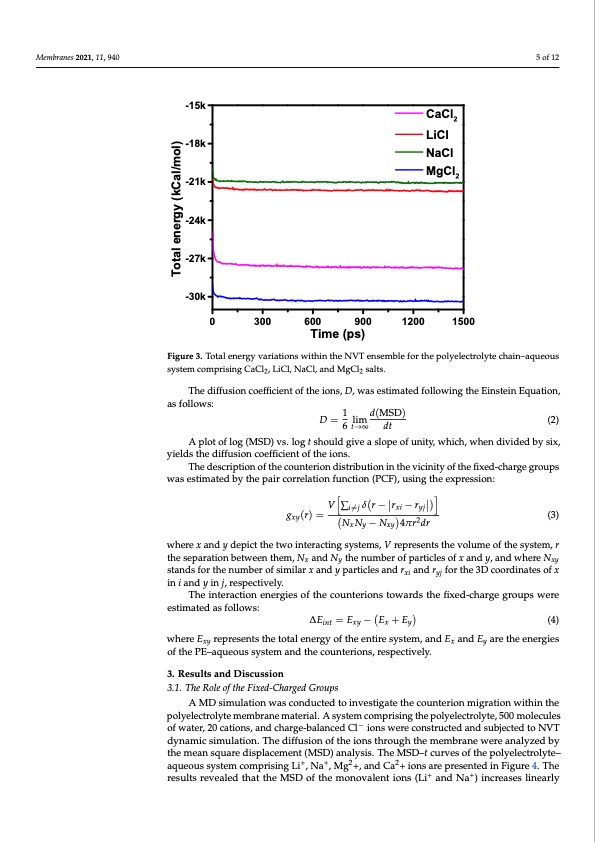
Membranes 2021, 11, 940 Membranes 2021, 11, 940 5 of 12 5 of 12 -15k -18k -21k -24k -27k -30k CaCl2 LiCl NaCl MgCl2 0 300 600 Time (ps) 900 1200 1500 Figure 3. Total energy variations within the NVT ensemble for the polyelectrolyte chain–aqueous systemcomprisingCaCl,LiCl,NaCl,andMgCl salts. Figure 3. Total energy variati2ons within the NVT ens2emble for the polyelectrolyte chain–aqueous system comprising CaCl2, LiCl, NaCl, and MgCl2 salts. The diffusion coefficient of the ions, D, was estimated following the Einstein Equation, as follows: The diffusion properties of the ions within the polyelectrolytes were determined by 1 d(MSD) estimating the mean square displacemeDnt=(MSDlim). The MSD indicates the average separa- (2) 6 t→∞ dt tion of all particles from their corresponding initial positions at time t into the motion. The A plot of log (MSD) vs. log t should give a slope of unity, which, when divided by six, greater the MSD value, the higher the diffusivity of the ions within the system. The MSD yields the diffusion coefficient of the ions. at time t in a given ensemble is expressed by the Equation: The description of the counterion distribution in the vicinity of the fixed-charge groups was estimated by the pair cMorSrDel=ationfunc|tio(n)(−PCF)(,0u)s|ing the expression: (1) 2 where N represents the number of particles to be averaged, and xi(0) and xi(t) are the initial V∑i̸=j δr − rxi − ryj positions of the i ion and the position of the ion at time t, respectively. gxy(r)= NN−N4πr2dr (3) xy xy The diffusion coefficient of the ions, D, was estimated following the Einstein Equa- tion, as follows: where x and y depict the two interacting systems, V represents the volume of the system, r 1 () the separation between them, Nx and Ny the number of particles of x and y, and where Nxy = lim (2) stands for the number of similar x and6y→particles and rxi and ryj for the 3D coordinates of x in i and y in j, respectively. A plot of log (MSD) vs. log t should give a slope of unity, which, when divided by The interaction energies of the counterions towards the fixed-charge groups were six, yields the diffusion coefficient of the ions. estimated as follows: The description of the counterion distribution in thevicinity of the fixed-charge ∆Eint=Exy− Ex+Ey (4) groups was estimated by the pair correlation function (PCF), using the expression: where E represents the total energy of the entire system, and E xy ∑ ( − − ) x and E where x and y depict the two interacting systems, V represents the volume of the system, of the PE–aqueous system a(nd) =the counterions, respectively. (3) r the separation between them, Nx and Ny the number of particles of x and y, and where 3. Results and Discussion 3.1. The Role of the Fixed-Charged Groups − 4 A MD simulation was conducted to investigate the counterion migration within the Nxy stands for the number of similar x and y particles and rxi and ryj for the 3D coordinates polyelectrolyte membrane material. A system comprising the polyelectrolyte, 500 molecules of x in i and y in j, respectively. of water, 20 cations, and charge-balanced Cl− ions were constructed and subjected to NVT The interaction energies of the counterions towards the fixed-charge groups were dynamic simulation. The diffusion of the ions through the membrane were analyzed by estimated as follows: the mean square displacement (MSD) analysis. The MSD–t curves of the polyelectrolyte– aqueous system comprising Li+, Na+, Mg2+, and Ca2+ ions are presented in Figure 4. The results revealed that the MSD of the monovalent ions (Li+ and Na+) increases linearly y are the energies Total energy (kCal/mol)PDF Image | Diffusion of Monovalent Ions in Polyelectrolyte
PDF Search Title:
Diffusion of Monovalent Ions in PolyelectrolyteOriginal File Name Searched:
membranes-11-00940-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |