
PDF Publication Title:
Text from PDF Page: 006
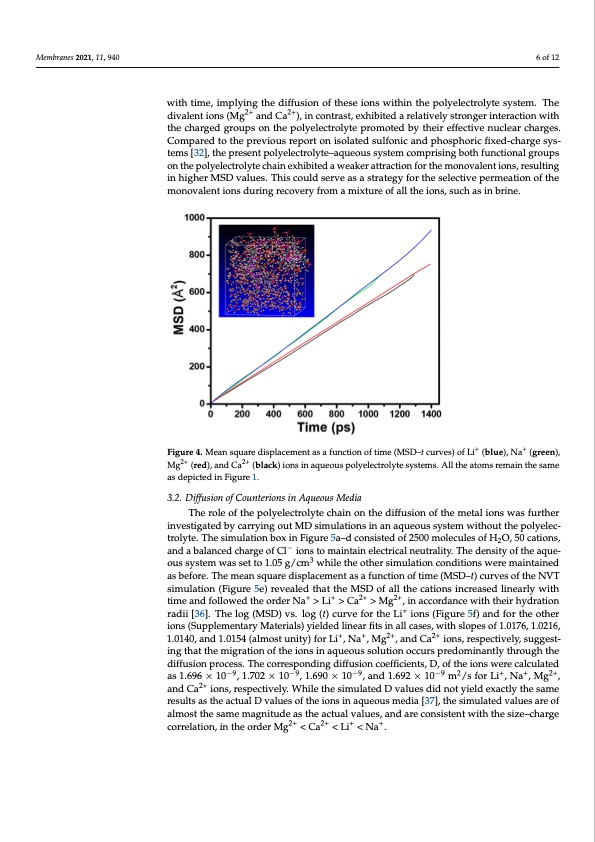
Membranes 2021, 11, 940 by the mean square displacement (MSD) analysis. The MSD–t curves of the polyelectro 6 of 12 polyelectrolyte membrane material. A system comprising the polyelectrolyte, 500 mole cules of water, 20 cations, and charge-balanced Cl- ions were constructed and subjected t NVT dynamic simulation. The diffusion of the ions through the membrane were analyze lyte–aqueous system comprising Li+, Na+, Mg2+, and Ca2+ ions are presented in Figure The results revealed that the MSD of the monovalent ions (Li+ and Na+) increases linearl with time, implying the diffusion of these ions within the polyelectrolyte system. The di with time, imply2i+ng the di2f+fusion of these ions within the polyelectrolyte system. The valent ions (Mg and Ca ), in contrast, exhibited a relatively stronger interaction with th divalent ions (Mg2+ and Ca2+), in contrast, exhibited a relatively stronger interaction with charged groups on the polyelectrolyte promoted by their effective nuclear charges. Com the charged groups on the polyelectrolyte promoted by their effective nuclear charges. pared to the previous report on isolated sulfonic and phosphoric fixed-charge system Compared to the previous report on isolated sulfonic and phosphoric fixed-charge sys- [32], the present polyelectrolyte–aqueous system comprising both functional groups o tems [32], the present polyelectrolyte–aqueous system comprising both functional groups the polyelectrolyte chain exhibited a weaker attraction for the monovalent ions, resultin on the polyelectrolyte chain exhibited a weaker attraction for the monovalent ions, resulting in higher MSD values. This could serve as a strategy for the selective permeation of th in higher MSD values. This could serve as a strategy for the selective permeation of the monovalent ions during recovery from a mixture of all the ions, such as in brine. monovalent ions during recovery from a mixture of all the ions, such as in brine. Figure 4. Mean square displacement as a function of time (MSD–t curves) of Li+ (blue), +Na+ (green),+ Figure4.Meansquaredisplacementasafunctionoftime(MSD–tcurves)ofLi (blue),Na (green Mg2+ (red), and Ca2+ (black) ions in aqueous polyelectrolyte systems. All the atoms remain the same Mg2+ (red), and Ca2+ (black) ions in aqueous polyelectrolyte systems. All the atoms remain the sam as depicted in Figure 1. as depicted in Figure 1. 3.2. Diffusion of Counterions in Aqueous Media 3.2. Diffusion of Counterions in Aqueous Media The role of the polyelectrolyte chain on the diffusion of the metal ions was further ous system was set to 1.05 g/c-m3 while the other simulation conditions were maintained and a balanced charge of Cl ions to maintain electrical neutrality. The density of the aque as before. The mean square displace3ment as a function of time (MSD–t) curves of the NVT ous system was set to 1.05 g/cm while the other simulation conditions were maintaine The role of the polyelectrolyte chain on the diffusion of the metal ions was furthe investigated by carrying out MD simulations in an aqueous system without the polyelec- tirnovlyetset.igTahtedsimbyulcaatirornyibnogx oinuFtiMguDres5iam–dulcaotniosinstseidnoafn25a0q0umeoulescuslyestoefmHwOit,h5o0uctatihoensp, olyelec 2 atrnodlyatbea.lTanhceesdimchuarlgaetiofnCbloxioinsFtoigmuraein5tai–ndelceocntrsicisatlendeuotfra2l5it0y0.TmheoldeecnuslietysoftHhe2Oaq,u5e0-cation − simulation (Figure 5e) revealed that the MSD of all the cations increased linearly with as before. The mean square displacement as a function of time (MSD–t) curves of the NV time and followed the order Na+ > Li+ > Ca2+ > Mg2+, in accordance with their hydration simulation (Figure 5e) revealed that the MSD of all the cations increased linearly with tim radii [36]. The log (MSD) vs. log (t) curve for the Li+ ions (Figure 5f) and for the other and followed the order Na+ > Li+ > Ca2+ > Mg2+, in accordance with their hydration radi ions (Supplementary Materials) yielded linear fits in all cases, with slopes of 1.0176, 1.0216, [36]. The log (MSD) vs. log (t) cur+ve fo+r the2+Li+ ions 2(+Figure 5f) and for the other ion 1.0140, and 1.0154 (almost unity) for Li , Na , Mg , and Ca ions, respectively, suggest- ing that the migration of the ions in aqueous solution occurs predominantly through the diffusion process. The corresponding diffusion coefficients, D, of the ions were calculated as 1.696 × 10−9, 1.702 × 10−9, 1.690 × 10−9, and 1.692 × 10−9 m2/s for Li+, Na+, Mg2+, and Ca2+ ions, respectively. While the simulated D values did not yield exactly the same results as the actual D values of the ions in aqueous media [37], the simulated values are of almost the same magnitude as the actual values, and are consistent with the size–charge correlation, in the order Mg2+ < Ca2+ < Li+ < Na+. o d 4 y e s n g e ) e s d T e sPDF Image | Diffusion of Monovalent Ions in Polyelectrolyte
PDF Search Title:
Diffusion of Monovalent Ions in PolyelectrolyteOriginal File Name Searched:
membranes-11-00940-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |