
PDF Publication Title:
Text from PDF Page: 008
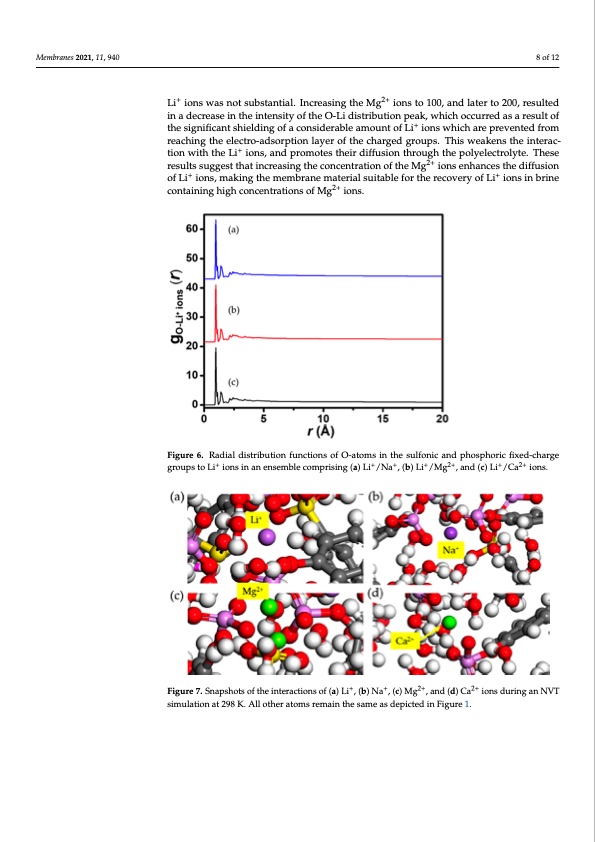
Membranes 2021, 11, 940 8 of 12 Membranes 2021, 11, 940 Membranes 2021, 11, 940 Li+ ions was not substantial. Increasing the Mg2+ ions to 100, and later to 200, resulted in a decrease in the intensity of the O-Li distribution peak, which occurred as a result of the significant shielding of a considerable amount of Li+ ions which are prevented from reaching the electro-adsorption layer of the charged groups. This weakens the interac- tion with the Li + 8 of 12 ions, and promotes their diffusion through the polyelectrolyte. These results suggest that increasing the concentration of the Mg2+ ions enhances the diffusion + 8 of 12 ions in brine + containing high concentrations of Mg2+ ions. of Li ions, making the membrane material suitable for the recovery of Li Figure 6. Radial distribution functions of O-atoms in the sulfonic and phosphoric fixed-charge Figure 6. Radial distribution functions of O-atoms in the sulfonic and phosphoric fixed-charge Figure 6. Radial distribution functions of O-atoms in the sulfonic and phosphoric fixed-charge ++ ++++++2+2+ +2+2+ ggrorouuppsstotoLLiiioinosnisninananenesnesmemblbelecocmomprpirsisnigng(a()aL)iL/iN/aN,a(b,)(Lbi)/LMig/M,agnd,(acn)dLi(c/C)Lai /ioCnas. ions. groups to Li+ ions in an ensemble comprising (a) Li+/Na+, (b) Li+/Mg2+, and (c) Li+/Ca2+ ions. + + 2+ 2+ Figure 7. Snapshots of the interactions of (a) Li+, (b) Na +, (c) Mg 2,+and (d) Ca 2+ions during an NVT Figure 7. Snapshots of the interactions of (a) Li , (b) Na , (c) Mg , and (d) Ca ions during an NVT simulation at 298 K. All other atoms remain the same as depicted in Figure 1. simulation at 298 K. All other atoms remain the same as depicted in Figure 1. Figure 7. Snapshots of the interactions of (a) Li+, (b) Na+, (c) Mg2+, and (d) Ca2+ ions during an NVT 3.4. Interaction of the Polyelectrolyte Chain with Mg2+/Li+ Ions simulation at 298 K. All other atoms remain the same as depicted in Figure 1. The concentration of Mg2+ ions in most brine is often greater than those of Li+ ions [40–42]. This results in a competition in the diffusion2+ of +the ions during the recovery pro- 3.4. Interaction of the Polyelectrolyte Chain with Mg /Li Ions cess. In order to suppress the interference of the Mg2+ ions, it is desired that the polyelec- The concentration of Mg2+ ions in most brine is often greater than those of Li+ ions trolyte chain in the membrane material exhibits a high affinity and, consequently, a [40–42]. This results in a competition in the diffusion of the ions during the recovery pro- greater retardation for the competing ions. The interaction of the polyelectrolyte chain cess. In or2d+ er+ to suppress the interference of the Mg2+ ions, it is desired that the polyelec- withMg /Li ionswasinvestigatedinthisstudytoexploretheeffectofahighconcentra- trolyte cha2i+n in the membrane ma+terial exhibits a high affinity and, consequently, a tion of Mg ions on the diffusion of Li ions. Three PE–aqueous systems were constructed, + grceoamteprrirseintagr5daptoiolynelefoctrrothlyeteccohmaipnes,ti2n5g00iomnosl.ecTuhlesionfteHr2aOct,i2o0nLoi fiothnes, p50o,ly10e0le, catnrdol2y0t0e chain 2+ 2+ + - w Mi t h g M i o g n s / L i n i t h i o e n f i s r s w t , a s s e c i o n n v d e , s a t n i g d a t t h e i d r d i n s y t s h t i e s m s s t , u r d e s y p t e o c t e i v x e p l l y o , r a e n d t h a e b e a f l f a e n c c t e o d f c a h a h r i g g e h o c f o C n l c e n t r a -PDF Image | Diffusion of Monovalent Ions in Polyelectrolyte
PDF Search Title:
Diffusion of Monovalent Ions in PolyelectrolyteOriginal File Name Searched:
membranes-11-00940-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |