
PDF Publication Title:
Text from PDF Page: 007
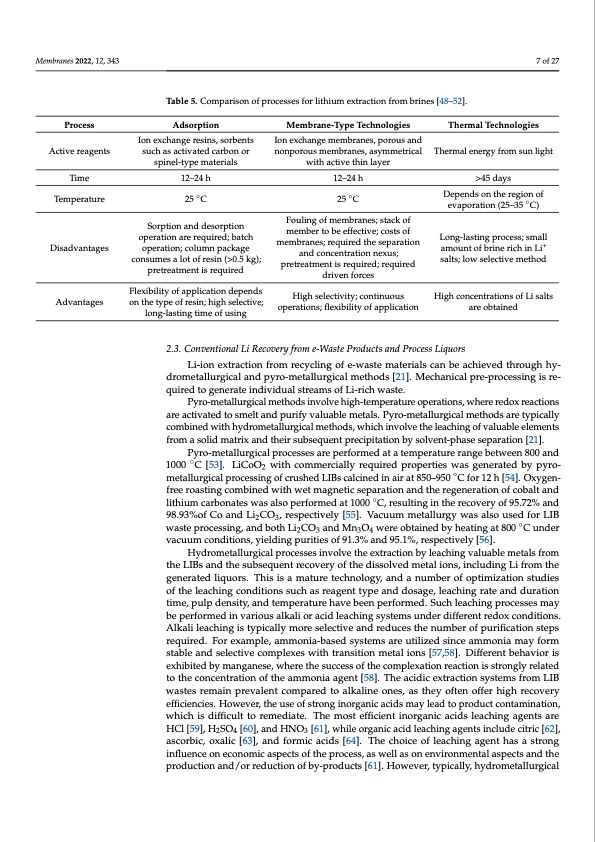
Membranes 2022, 12, 343 7 of 27 Table 5. Comparison of processes for lithium extraction from brines [48–52]. Process Active reagents Time Temperature Disadvantages Advantages Adsorption Ion exchange resins, sorbents such as activated carbon or spinel-type materials 12–24 h 25 ◦C Sorption and desorption operation are required; batch operation; column package consumes a lot of resin (>0.5 kg); pretreatment is required Flexibility of application depends on the type of resin; high selective; long-lasting time of using Membrane-Type Technologies Ion exchange membranes, porous and nonporous membranes, asymmetrical with active thin layer 12–24 h 25 ◦C Fouling of membranes; stack of member to be effective; costs of membranes; required the separation and concentration nexus; pretreatment is required; required driven forces High selectivity; continuous operations; flexibility of application Thermal Technologies Thermal energy from sun light >45 days Depends on the region of evaporation (25–35 ◦C) Long-lasting process; small amount of brine rich in Li+ salts; low selective method High concentrations of Li salts are obtained 2.3. Conventional Li Recovery from e-Waste Products and Process Liquors Li-ion extraction from recycling of e-waste materials can be achieved through hy- drometallurgical and pyro-metallurgical methods [21]. Mechanical pre-processing is re- quired to generate individual streams of Li-rich waste. Pyro-metallurgical methods involve high-temperature operations, where redox reactions are activated to smelt and purify valuable metals. Pyro-metallurgical methods are typically combined with hydrometallurgical methods, which involve the leaching of valuable elements from a solid matrix and their subsequent precipitation by solvent-phase separation [21]. Pyro-metallurgical processes are performed at a temperature range between 800 and 1000 ◦C [53]. LiCoO2 with commercially required properties was generated by pyro- metallurgical processing of crushed LIBs calcined in air at 850–950 ◦C for 12 h [54]. Oxygen- free roasting combined with wet magnetic separation and the regeneration of cobalt and lithium carbonates was also performed at 1000 ◦C, resulting in the recovery of 95.72% and 98.93%of Co and Li2CO3, respectively [55]. Vacuum metallurgy was also used for LIB waste processing, and both Li2CO3 and Mn3O4 were obtained by heating at 800 ◦C under vacuum conditions, yielding purities of 91.3% and 95.1%, respectively [56]. Hydrometallurgical processes involve the extraction by leaching valuable metals from the LIBs and the subsequent recovery of the dissolved metal ions, including Li from the generated liquors. This is a mature technology, and a number of optimization studies of the leaching conditions such as reagent type and dosage, leaching rate and duration time, pulp density, and temperature have been performed. Such leaching processes may be performed in various alkali or acid leaching systems under different redox conditions. Alkali leaching is typically more selective and reduces the number of purification steps required. For example, ammonia-based systems are utilized since ammonia may form stable and selective complexes with transition metal ions [57,58]. Different behavior is exhibited by manganese, where the success of the complexation reaction is strongly related to the concentration of the ammonia agent [58]. The acidic extraction systems from LIB wastes remain prevalent compared to alkaline ones, as they often offer high recovery efficiencies. However, the use of strong inorganic acids may lead to product contamination, which is difficult to remediate. The most efficient inorganic acids leaching agents are HCl [59], H2SO4 [60], and HNO3 [61], while organic acid leaching agents include citric [62], ascorbic, oxalic [63], and formic acids [64]. The choice of leaching agent has a strong influence on economic aspects of the process, as well as on environmental aspects and the production and/or reduction of by-products [61]. However, typically, hydrometallurgicalPDF Image | Electro-Driven Materials and Processes for Lithium
PDF Search Title:
Electro-Driven Materials and Processes for LithiumOriginal File Name Searched:
membranes-12-00343-v3.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |