
PDF Publication Title:
Text from PDF Page: 009
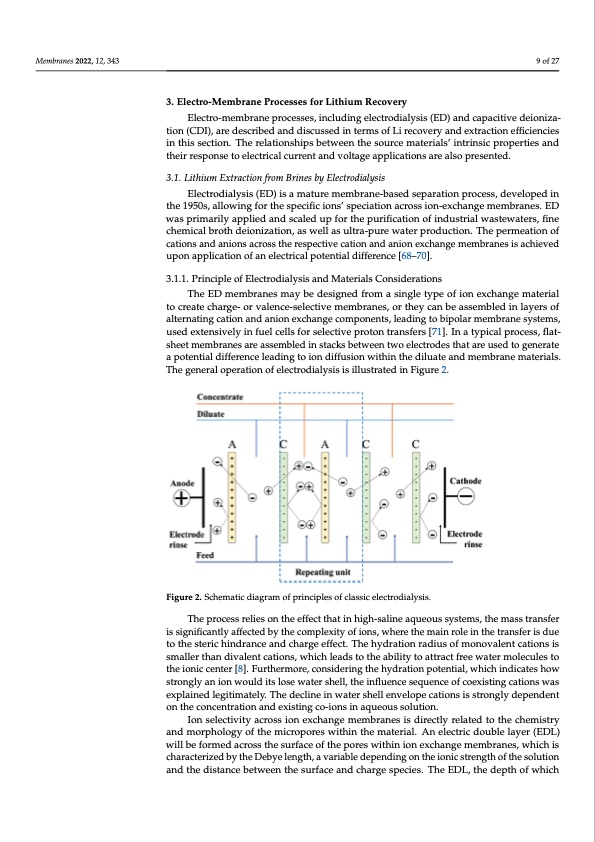
Membranes 2022, 12, 343 9 of 27 3. Electro-Membrane Processes for Lithium Recovery Electro-membrane processes, including electrodialysis (ED) and capacitive deioniza- tion (CDI), are described and discussed in terms of Li recovery and extraction efficiencies in this section. The relationships between the source materials’ intrinsic properties and their response to electrical current and voltage applications are also presented. 3.1. Lithium Extraction from Brines by Electrodialysis Electrodialysis (ED) is a mature membrane-based separation process, developed in the 1950s, allowing for the specific ions’ speciation across ion-exchange membranes. ED was primarily applied and scaled up for the purification of industrial wastewaters, fine chemical broth deionization, as well as ultra-pure water production. The permeation of cations and anions across the respective cation and anion exchange membranes is achieved upon application of an electrical potential difference [68–70]. 3.1.1. Principle of Electrodialysis and Materials Considerations The ED membranes may be designed from a single type of ion exchange material to create charge- or valence-selective membranes, or they can be assembled in layers of Membranes 2022, 11, x FOR PEER REVIEW 9 of 27 alternating cation and anion exchange components, leading to bipolar membrane systems, used extensively in fuel cells for selective proton transfers [71]. In a typical process, flat- sheet membranes are assembled in stacks between two electrodes that are used to generate a potential difference leading to ion diffusion within the diluate and membrane materials. a potential difference leading to ion diffusion within the diluate and membrane materials. The general operation of electrodialysis is illustrated in Figure 2. The general operation of electrodialysis is illustrated in Figure 2. Figure 2. Schematic diagram of principles of classic electrodialysis. Figure 2. Schematic diagram of principles of classic electrodialysis. Theprroccessrrelliiesonttheeefffecctttthattiinhiiggh--ssaalliineeaaqueeoussssysstteemss,,ttheemaasssttrraanssffeerr iisssiigniffiicantly affectedbytthheeccoompplelxexitiytyofoifoinosn,sw, whehrertehethmeaminairnolreoilnetihnetthreantsrfaenrsifserduise dtouethtoetshtersictehricndhrinadnrcaenacnedanchdacrhgaeregfefecftf.ecTth.Teheyhdyradtriaotniornadraiudsiuosfomfomnonvoavleanletnctactaiotinosniss ismsmalalellretrhtahnandidviavlaelnetnctactaiotinosn,sw,whihchichlealedasdtsotothtehaebaibliitlyitytotaottartatrcatcftrefreeweawteartemromleocleucluesletso ttohethieonioicncicencteenrte[8r][.8F]u. Frtuhretrhmeromreo,rceo,ncsoidnesirdinegritnhgethyedhryatdiorantipoontepnottieanl,twiahl,icwhhiincdhicinatdeiscahtoews hsotrwonsgtrlyonagnlyioannwioonuwldoiutsldloistse lwosaetewr astheerlsl,htehlel, itnhfleuinenflcueesnecqeusenqcueeonfcecoefxciosteixnigstcinatgiocnatsiownass explained legitimately. The decline in water shell envelope cations is strongly dependent was explained legitimately. The decline in water shell envelope cations is strongly de- on the concentration and existing co-ions in aqueous solution. pendent on the concentration and existing co-ions in aqueous solution. Ion selectivity across ion exchange membranes is directly related to the chemistry Ion selectivity across ion exchange membranes is directly related to the chemistry and morphology of the micropores within the material. An electric double layer (EDL) and morphology of the micropores within the material. An electric double layer (EDL) will be formed across the surface of the pores within ion exchange membranes, which is will be formed across the surface of the pores within ion exchange membranes, which is characterized by the Debye length, a variable depending on the ionic strength of the solution characterized by the Debye length, a variable depending on the ionic strength of the solu- and the distance between the surface and charge species. The EDL, the depth of which tion and the distance between the surface and charge species. The EDL, the depth of which may vary from ~1 nm to a few tens of nano-meters, consists of the Stern and Helmholtz layers, which correspond to either polarized or diffuse layers, respectively [72]. The elec- trical attraction generated by the diffuse layer is weaker than that of the polarized layer, which means that counter ions can diffuse with limited resistances. These interactions are typically measured in terms of the streaming or zeta potential, which decay exponentiallyPDF Image | Electro-Driven Materials and Processes for Lithium
PDF Search Title:
Electro-Driven Materials and Processes for LithiumOriginal File Name Searched:
membranes-12-00343-v3.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |