
PDF Publication Title:
Text from PDF Page: 003
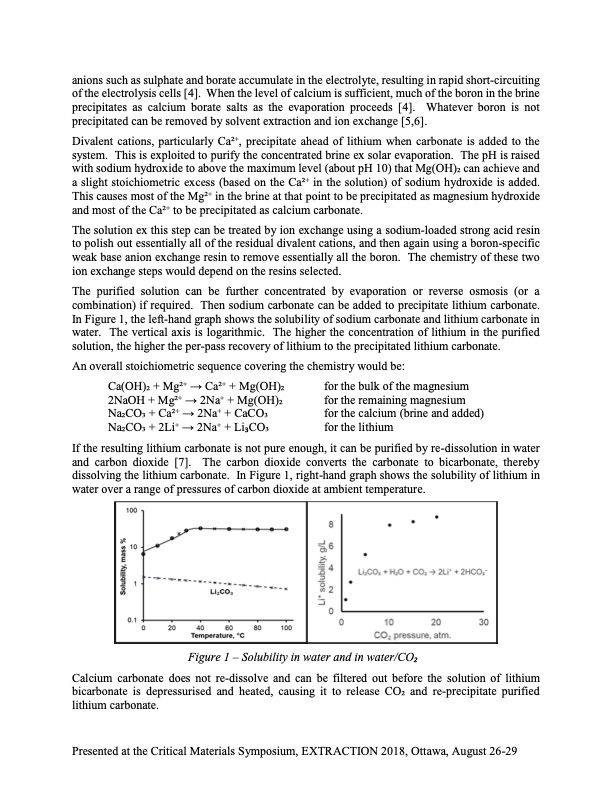
anions such as sulphate and borate accumulate in the electrolyte, resulting in rapid short-circuiting of the electrolysis cells [4]. When the level of calcium is sufficient, much of the boron in the brine precipitates as calcium borate salts as the evaporation proceeds [4]. Whatever boron is not precipitated can be removed by solvent extraction and ion exchange [5,6]. Divalent cations, particularly Ca2+, precipitate ahead of lithium when carbonate is added to the system. This is exploited to purify the concentrated brine ex solar evaporation. The pH is raised with sodium hydroxide to above the maximum level (about pH 10) that Mg(OH)2 can achieve and a slight stoichiometric excess (based on the Ca2+ in the solution) of sodium hydroxide is added. This causes most of the Mg2+ in the brine at that point to be precipitated as magnesium hydroxide and most of the Ca2+ to be precipitated as calcium carbonate. The solution ex this step can be treated by ion exchange using a sodium-loaded strong acid resin to polish out essentially all of the residual divalent cations, and then again using a boron-specific weak base anion exchange resin to remove essentially all the boron. The chemistry of these two ion exchange steps would depend on the resins selected. The purified solution can be further concentrated by evaporation or reverse osmosis (or a combination) if required. Then sodium carbonate can be added to precipitate lithium carbonate. In Figure 1, the left-hand graph shows the solubility of sodium carbonate and lithium carbonate in water. The vertical axis is logarithmic. The higher the concentration of lithium in the purified solution, the higher the per-pass recovery of lithium to the precipitated lithium carbonate. An overall stoichiometric sequence covering the chemistry would be: Ca(OH)2 + Mg2+ → Ca2+ + Mg(OH)2 2NaOH + Mg2+ → 2Na+ + Mg(OH)2 Na2CO3 + Ca2+ → 2Na+ + CaCO3 Na2CO3 + 2Li+ → 2Na+ + Li3CO3 for the bulk of the magnesium for the remaining magnesium for the calcium (brine and added) for the lithium If the resulting lithium carbonate is not pure enough, it can be purified by re-dissolution in water and carbon dioxide [7]. The carbon dioxide converts the carbonate to bicarbonate, thereby dissolving the lithium carbonate. In Figure 1, right-hand graph shows the solubility of lithium in water over a range of pressures of carbon dioxide at ambient temperature. Figure 1 – Solubility in water and in water/CO2 Calcium carbonate does not re-dissolve and can be filtered out before the solution of lithium bicarbonate is depressurised and heated, causing it to release CO2 and re-precipitate purified lithium carbonate. Presented at the Critical Materials Symposium, EXTRACTION 2018, Ottawa, August 26-29PDF Image | Extraction of Lithium from Brine Chemistry
PDF Search Title:
Extraction of Lithium from Brine ChemistryOriginal File Name Searched:
Extraction of Lithium from Brine 13 Old and New Chemistry.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |