
PDF Publication Title:
Text from PDF Page: 009
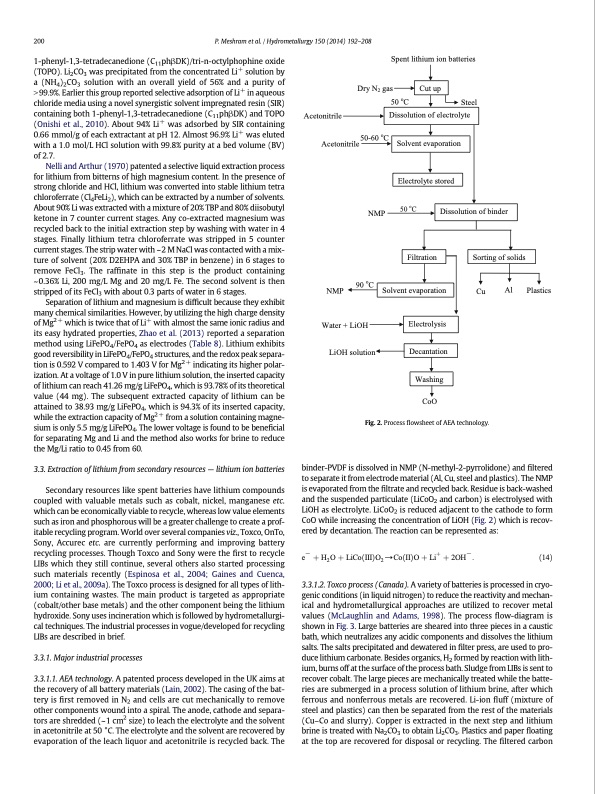
200 P. Meshram et al. / Hydrometallurgy 150 (2014) 192–208 1-phenyl-1,3-tetradecanedione (C11phβDK)/tri-n-octylphophine oxide (TOPO). Li2CO3 was precipitated from the concentrated Li+ solution by a (NH4)2CO3 solution with an overall yield of 56% and a purity of N 99.9%. Earlier this group reported selective adsorption of Li+ in aqueous chloride media using a novel synergistic solvent impregnated resin (SIR) containing both 1-phenyl-1,3-tetradecanedione (C11phβDK) and TOPO (Onishi et al., 2010). About 94% Li+ was adsorbed by SIR containing 0.66 mmol/g of each extractant at pH 12. Almost 96.9% Li+ was eluted with a 1.0 mol/L HCl solution with 99.8% purity at a bed volume (BV) of 2.7. Nelli and Arthur (1970) patented a selective liquid extraction process for lithium from bitterns of high magnesium content. In the presence of strong chloride and HCl, lithium was converted into stable lithium tetra chloroferrate (Cl4FeLi2), which can be extracted by a number of solvents. About 90% Li was extracted with a mixture of 20% TBP and 80% diisobutyl ketone in 7 counter current stages. Any co-extracted magnesium was recycled back to the initial extraction step by washing with water in 4 stages. Finally lithium tetra chloroferrate was stripped in 5 counter current stages. The strip water with ~ 2 M NaCl was contacted with a mix- ture of solvent (20% D2EHPA and 30% TBP in benzene) in 6 stages to remove FeCl3. The raffinate in this step is the product containing ~0.36% Li, 200 mg/L Mg and 20 mg/L Fe. The second solvent is then stripped of its FeCl3 with about 0.3 parts of water in 6 stages. Separation of lithium and magnesium is difficult because they exhibit many chemical similarities. However, by utilizing the high charge density of Mg2 + which is twice that of Li+ with almost the same ionic radius and its easy hydrated properties, Zhao et al. (2013) reported a separation method using LiFePO4/FePO4 as electrodes (Table 8). Lithium exhibits good reversibility in LiFePO4/FePO4 structures, and the redox peak separa- tion is 0.592 V compared to 1.403 V for Mg2 + indicating its higher polar- ization. At a voltage of 1.0 V in pure lithium solution, the inserted capacity of lithium can reach 41.26 mg/g LiFePO4, which is 93.78% of its theoretical value (44 mg). The subsequent extracted capacity of lithium can be attained to 38.93 mg/g LiFePO4, which is 94.3% of its inserted capacity, while the extraction capacity of Mg2 + from a solution containing magne- sium is only 5.5 mg/g LiFePO4. The lower voltage is found to be beneficial for separating Mg and Li and the method also works for brine to reduce the Mg/Li ratio to 0.45 from 60. 3.3. Extraction of lithium from secondary resources — lithium ion batteries Secondary resources like spent batteries have lithium compounds coupled with valuable metals such as cobalt, nickel, manganese etc. which can be economically viable to recycle, whereas low value elements such as iron and phosphorous will be a greater challenge to create a prof- itable recycling program. World over several companies viz., Toxco, OnTo, Sony, Accurec etc. are currently performing and improving battery recycling processes. Though Toxco and Sony were the first to recycle LIBs which they still continue, several others also started processing such materials recently (Espinosa et al., 2004; Gaines and Cuenca, 2000; Li et al., 2009a). The Toxco process is designed for all types of lith- ium containing wastes. The main product is targeted as appropriate (cobalt/other base metals) and the other component being the lithium hydroxide. Sony uses incineration which is followed by hydrometallurgi- cal techniques. The industrial processes in vogue/developed for recycling LIBs are described in brief. 3.3.1. Major industrial processes 3.3.1.1. AEA technology. A patented process developed in the UK aims at the recovery of all battery materials (Lain, 2002). The casing of the bat- tery is first removed in N2 and cells are cut mechanically to remove other components wound into a spiral. The anode, cathode and separa- tors are shredded (~1 cm2 size) to leach the electrolyte and the solvent in acetonitrile at 50 °C. The electrolyte and the solvent are recovered by evaporation of the leach liquor and acetonitrile is recycled back. The Spent lithium ion batteries Acetonitrile Acetonitrile 50-60 oC 50 oC Filtration Decantation WasChoiOng CoO Dry N2 gas 50 oC Cut up Dissolution of electrolyte Steel Solvent evaporation Electrolyte stored NMP Dissolution of binder NMP 90 oC Sorting of solids Cu Al Plastics Solvent evaporation Water + LiOH Electrolysis LiOH solution Fig. 2. Process flowsheet of AEA technology. binder-PVDF is dissolved in NMP (N-methyl-2-pyrrolidone) and filtered to separate it from electrode material (Al, Cu, steel and plastics). The NMP is evaporated from the filtrate and recycled back. Residue is back-washed and the suspended particulate (LiCoO2 and carbon) is electrolysed with LiOH as electrolyte. LiCoO2 is reduced adjacent to the cathode to form CoO while increasing the concentration of LiOH (Fig. 2) which is recov- ered by decantation. The reaction can be represented as: e− þ H2 O þ LiCoðIIIÞO2 →CoðIIÞO þ Liþ þ 2OH− : ð14Þ 3.3.1.2. Toxco process (Canada). A variety of batteries is processed in cryo- genic conditions (in liquid nitrogen) to reduce the reactivity and mechan- ical and hydrometallurgical approaches are utilized to recover metal values (McLaughlin and Adams, 1998). The process flow-diagram is shown in Fig. 3. Large batteries are sheared into three pieces in a caustic bath, which neutralizes any acidic components and dissolves the lithium salts. The salts precipitated and dewatered in filter press, are used to pro- duce lithium carbonate. Besides organics, H2 formed by reaction with lith- ium, burns off at the surface of the process bath. Sludge from LIBs is sent to recover cobalt. The large pieces are mechanically treated while the batte- ries are submerged in a process solution of lithium brine, after which ferrous and nonferrous metals are recovered. Li-ion fluff (mixture of steel and plastics) can then be separated from the rest of the materials (Cu–Co and slurry). Copper is extracted in the next step and lithium brine is treated with Na2CO3 to obtain Li2CO3. Plastics and paper floating at the top are recovered for disposal or recycling. The filtered carbonPDF Image | Extraction of lithium from primary and secondary sources
PDF Search Title:
Extraction of lithium from primary and secondary sourcesOriginal File Name Searched:
1ca41gb-Pratima-BDP-Li Review-Hydrom-2014.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |