
PDF Publication Title:
Text from PDF Page: 010
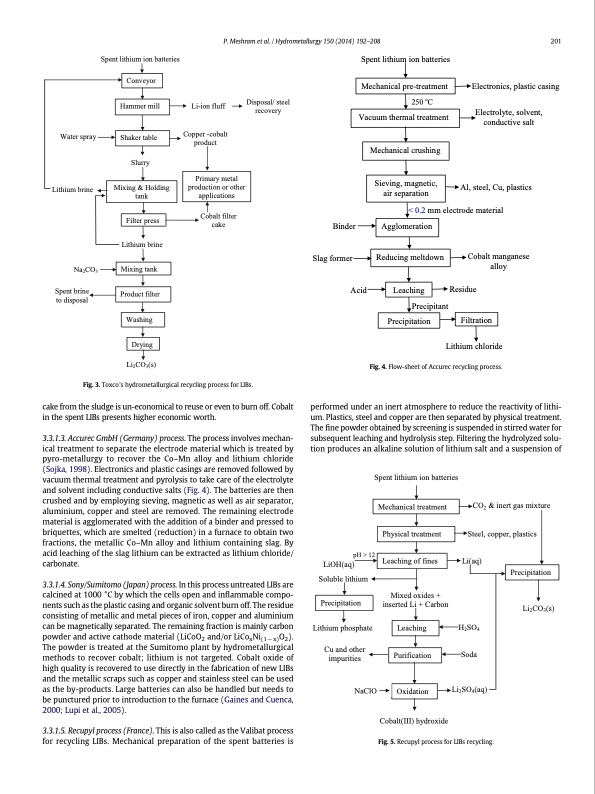
Spent lithium ion batteries Conveyor Hammer mill Shaker table Slurry Lithium brine Mixing tank Product filter Washing Drying Li2CO3(s) P. Meshram et al. / Hydrometallurgy 150 (2014) 192–208 201 Spent lithium ion batteries Mechanical pre-treatment 250 oC Li-ion fluff Disposal/ steel recovery Electronics, plastic casing Al, steel, Cu, plastics Vacuum thermal treatment Electrolyte, solvent, conductive salt Water spray Lithium brine Na2CO3 Mechanical crushing Copper -cobalt product Primary metal production or other applications Sieving, magnetic, air separation Mixing & Holding tank Filter press Cobalt filter cake Binder Slag former Acid < 0.2 mm electrode material Agglomeration Reducing meltdown Lithium chloride Fig. 4. Flow-sheet of Accurec recycling process. Cobalt manganese alloy Spent brine to disposal Leaching Residue Precipitant Precipitation Filtration Fig. 3. Toxco's hydrometallurgical recycling process for LIBs. cake from the sludge is un-economical to reuse or even to burn off. Cobalt in the spent LIBs presents higher economic worth. 3.3.1.3. Accurec GmbH (Germany) process. The process involves mechan- ical treatment to separate the electrode material which is treated by pyro-metallurgy to recover the Co–Mn alloy and lithium chloride (Sojka, 1998). Electronics and plastic casings are removed followed by vacuum thermal treatment and pyrolysis to take care of the electrolyte and solvent including conductive salts (Fig. 4). The batteries are then crushed and by employing sieving, magnetic as well as air separator, aluminium, copper and steel are removed. The remaining electrode material is agglomerated with the addition of a binder and pressed to briquettes, which are smelted (reduction) in a furnace to obtain two fractions, the metallic Co–Mn alloy and lithium containing slag. By acid leaching of the slag lithium can be extracted as lithium chloride/ carbonate. 3.3.1.4. Sony/Sumitomo (Japan) process. In this process untreated LIBs are calcined at 1000 °C by which the cells open and inflammable compo- nents such as the plastic casing and organic solvent burn off. The residue consisting of metallic and metal pieces of iron, copper and aluminium can be magnetically separated. The remaining fraction is mainly carbon powder and active cathode material (LiCoO2 and/or LiCoxNi(1−x)O2). The powder is treated at the Sumitomo plant by hydrometallurgical methods to recover cobalt; lithium is not targeted. Cobalt oxide of high quality is recovered to use directly in the fabrication of new LIBs and the metallic scraps such as copper and stainless steel can be used as the by-products. Large batteries can also be handled but needs to be punctured prior to introduction to the furnace (Gaines and Cuenca, 2000; Lupi et al., 2005). 3.3.1.5. Recupyl process (France). This is also called as the Valibat process for recycling LIBs. Mechanical preparation of the spent batteries is performed under an inert atmosphere to reduce the reactivity of lithi- um. Plastics, steel and copper are then separated by physical treatment. The fine powder obtained by screening is suspended in stirred water for subsequent leaching and hydrolysis step. Filtering the hydrolyzed solu- tion produces an alkaline solution of lithium salt and a suspension of pH > 12 LiOH(aq) Soluble lithium Lithium phosphate Li2CO3(s) Spent lithium ion batteries Mechanical treatment Physical treatment Leaching of fines Leaching Purification Cobalt(III) hydroxide CO2 & inert gas mixture Steel, copper, plastics Li(aq) Precipitation Mixed oxides + inserted Li + Carbon Precipitation H2SO4 Soda Cu and other impurities NaClO Oxidation Fig. 5. Recupyl process for LIBs recycling. Li2SO4(aq)PDF Image | Extraction of lithium from primary and secondary sources
PDF Search Title:
Extraction of lithium from primary and secondary sourcesOriginal File Name Searched:
1ca41gb-Pratima-BDP-Li Review-Hydrom-2014.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |