
PDF Publication Title:
Text from PDF Page: 012
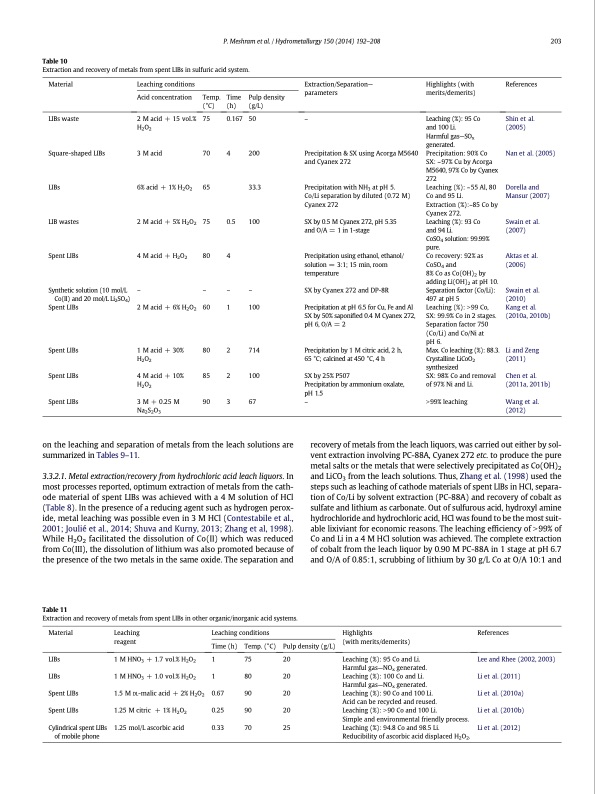
Table 10 Extraction and recovery of metals from spent LIBs in sulfuric acid system. P. Meshram et al. / Hydrometallurgy 150 (2014) 192–208 203 Material LIBs waste Square-shaped LIBs LIBs LIB wastes Spent LIBs Synthetic solution (10 mol/L Co(II) and 20 mol/L Li2SO4) Spent LIBs Spent LIBs Spent LIBs Spent LIBs Leaching conditions Acid concentration Temp. (°C) Extraction/Separation— parameters – Precipitation & SX using Acorga M5640 and Cyanex 272 Precipitation with NH3 at pH 5. Co/Li separation by diluted (0.72 M) Cyanex 272 SX by 0.5 M Cyanex 272, pH 5.35 and O/A = 1 in 1-stage Precipitation using ethanol, ethanol/ solution = 3:1; 15 min, room temperature SX by Cyanex 272 and DP-8R Precipitation at pH 6.5 for Cu, Fe and Al SX by 50% saponified 0.4 M Cyanex 272, pH 6, O/A = 2 Precipitation by 1 M citric acid, 2 h, 65 °C; calcined at 450 °C, 4 h SX by 25% P507 Precipitation by ammonium oxalate, pH 1.5 – Highlights (with merits/demerits) Leaching (%): 95 Co and 100 Li. Harmful gas—SOx generated. Precipitation: 90% Co SX: ~97% Cu by Acorga M5640, 97% Co by Cyanex 272 Leaching (%): ~55 Al, 80 Co and 95 Li. Extraction (%):~85 Co by Cyanex 272. Leaching (%): 93 Co and 94 Li. CoSO4 solution: 99.99% pure. Co recovery: 92% as CoSO4 and 8% Co as Co(OH)2 by adding Li(OH)2 at pH 10. Separation factor (Co/Li): 497 at pH 5 Leaching (%): N99 Co, SX: 99.9% Co in 2 stages. Separation factor 750 (Co/Li) and Co/Ni at pH 6. Max. Co leaching (%): 88.3. Crystalline LiCoO2 synthesized SX: 98% Co and removal of 97% Ni and Li. N99% leaching References Shin et al. (2005) Nan et al. (2005) Dorella and Mansur (2007) Swain et al. (2007) Aktas et al. (2006) Swain et al. (2010) Kang et al. (2010a, 2010b) Li and Zeng (2011) Chen et al. (2011a, 2011b) Wang et al. (2012) 2Macid+15vol.% 75 H2O2 3 M acid 70 6% acid + 1% H2O2 65 2 Macid+5%H2O2 75 4 M acid + H2O2 80 – – 2 Macid+6%H2O2 60 1 Macid+30% 80 H2O2 4 Macid + 10% 85 H2O2 3 M + 0.25 M 90 Na2S2O3 Time Pulp density (h) (g/L) 0.167 50 4 200 33.3 0.5 100 4 – – 1 100 2 714 2 100 3 67 the leach solutions are on the leaching and separation of metals from summarized in Tables 9–11. recovery of metals from the leach liquors, was carried out either by sol- vent extraction involving PC-88A, Cyanex 272 etc. to produce the pure metal salts or the metals that were selectively precipitated as Co(OH)2 and LiCO3 from the leach solutions. Thus, Zhang et al. (1998) used the steps such as leaching of cathode materials of spent LIBs in HCl, separa- tion of Co/Li by solvent extraction (PC-88A) and recovery of cobalt as sulfate and lithium as carbonate. Out of sulfurous acid, hydroxyl amine hydrochloride and hydrochloric acid, HCl was found to be the most suit- able lixiviant for economic reasons. The leaching efficiency of N99% of Co and Li in a 4 M HCl solution was achieved. The complete extraction of cobalt from the leach liquor by 0.90 M PC-88A in 1 stage at pH 6.7 and O/A of 0.85:1, scrubbing of lithium by 30 g/L Co at O/A 10:1 and 3.3.2.1. Metal extraction/recovery from hydrochloric acid leach liquors. In most processes reported, optimum extraction of metals from the cath- ode material of spent LIBs was achieved with a 4 M solution of HCl (Table 8). In the presence of a reducing agent such as hydrogen perox- ide, metal leaching was possible even in 3 M HCl (Contestabile et al., 2001; Joulié et al., 2014; Shuva and Kurny, 2013; Zhang et al, 1998). While H2O2 facilitated the dissolution of Co(II) which was reduced from Co(III), the dissolution of lithium was also promoted because of the presence of the two metals in the same oxide. The separation and Table 11 Extraction and recovery of metals from spent LIBs in other organic/inorganic acid systems. Material LIBs LIBs Spent LIBs Spent LIBs Cylindrical spent LIBs of mobile phone Leaching reagent 1 M HNO3 + 1.7 vol.% H2O2 1 M HNO3 + 1.0 vol.% H2O2 1.5 M DL-malic acid + 2% H2O2 1.25 M citric + 1% H2O2 1.25 mol/L ascorbic acid Leaching conditions Highlights References (with merits/demerits) Time (h) 1 1 0.67 0.25 0.33 Temp. (°C) Pulp density (g/L) 75 20 80 20 90 20 90 20 70 25 Leaching (%): 95 Co and Li. Harmful gas—NOx generated. Leaching (%): 100 Co and Li. Harmful gas—NOx generated. Leaching (%): 90 Co and 100 Li. Acid can be recycled and reused. Leaching (%): N90 Co and 100 Li. Simple and environmental friendly process. Leaching (%): 94.8 Co and 98.5 Li. Reducibility of ascorbic acid displaced H2O2. Lee and Rhee (2002, 2003) Li et al. (2011) Li et al. (2010a) Li et al. (2010b) Li et al. (2012)PDF Image | Extraction of lithium from primary and secondary sources
PDF Search Title:
Extraction of lithium from primary and secondary sourcesOriginal File Name Searched:
1ca41gb-Pratima-BDP-Li Review-Hydrom-2014.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |