
PDF Publication Title:
Text from PDF Page: 005
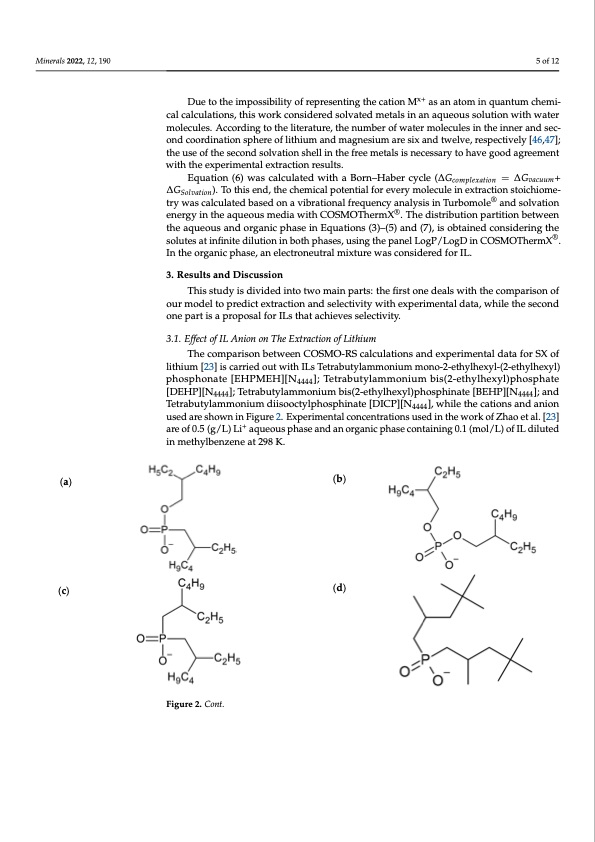
Minerals 2022, 12, 190 force field level of theory, which is implemented in the PCmodel software [45]. Only mol ecules with the lowest energy and not exceeding an energy threshold by more than 0. kcal/mol were reoptimized in Turbomole®, and then the molecules with the lowest energ (potential chemical in vacuum + solvation free energy) were employed. 5 of 12 Due to the impossibility of representing the cation Mx+ as an atom in quantum chem ical calculations, this work considered solvated metals in an aqueous solution with wate x+ mDuoeletcoutlhees.imApccoossridbinligtytof trheperleistenratitnugret,hethceatniounmMber aosfawnateormminolqeucaunletusminchtheemin- ner an cal calculations, this work considered solvated metals in an aqueous solution with water second coordination sphere of lithium and magnesium are six and twelve, respectivel molecules. According to the literature, the number of water molecules in the inner and sec- [46,47]; the use of the second solvation shell in the free metals is necessary to have goo ond coordination sphere of lithium and magnesium are six and twelve, respectively [46,47]; agreement with the experimental extraction results. the use of the second solvation shell in the free metals is necessary to have good agreement Equation (6) was calculated with a Born–Haber cycle ( ∆𝐺 with the experimental extraction results. ∆𝐺+∆𝐺). To this end, the chemical potential for every molecule in extractio Equation (6) was calculated with a Born–Haber cycle (∆Gcomplexation = ∆Gvacuum+ stoichiometry was calculated based on a vibrational frequency analysis in Turbomole ∆GSolvation). To this end, the chemical potential for every molecule in extraction stoichiome- and solvation energy in the aqueous media with COSMOThermX®. The distribution par try was calculated based on a vibrational frequency analysis in Turbomole® and solvation tition between the aqueous and organic pha®se in Equations (3)–(5) and (7), is obtaine energyintheaqueousmediawithCOSMOThermX .Thedistributionpartitionbetween considering the solutes at infinite dilution in both phases, using the panel LogP/LogD i the aqueous and organic phase in Equations (3)–(5) and (7), is obtained considering the COSMOThermX®. In the organic phase, an electroneutral mixture was considered for IL. solutes at infinite dilution in both phases, using the panel LogP/LogD in COSMOThermX®. In the organic phase, an electroneutral mixture was considered for IL. 3. Results and Discussion 3. Results and Discussion This study is divided into two main parts: the first one deals with the comparison o This study is divided into two main parts: the first one deals with the comparison of our model to predict extraction and selectivity with experimental data, while the secon our model to predict extraction and selectivity with experimental data, while the second one part is a proposal for ILs that achieves selectivity. one part is a proposal for ILs that achieves selectivity. 3.1. Effect of IL Anion on The Extraction of Lithium 3.1. Effect of IL Anion on The Extraction of Lithium The comparison between COSMO-RS calculations and experimental data for SX o The comparison between COSMO-RS calculations and experimental data for SX of lithium [23] is carried out with ILs Tetrabutylammonium mono-2-ethylhexyl-(2 lithium [23] is carried out with ILs Tetrabutylammonium mono-2-ethylhexyl-(2-ethylhexyl) ethylhexyl) phosphonate [EHPMEH][N4444]; Tetrabutylammonium bis(2-ethylhexyl)phos phosphonate [EHPMEH][N4444]; Tetrabutylammonium bis(2-ethylhexyl)phosphate (a) (c) IL diluted in methylbenzene at 298 K. (b) [DEHpPh]a[tNe [DE];HTePt]r[aNb4u44t4y]l;aTmemtraobnuiutmylabmism(2-oenthiuymlhebxiys(l)2p-ehtohsyplhienxaytel)p[BhEoHspPh][iNnate ][;BaEnHdP][N4444 4444 4444 TetraabnudtyTlaemtrmabountiyulmamdmiisoonoicutmylpdhiiossopohcitnyalpteh[oDsIpChPin][aNte [D]I,CwPh]i[lNe 4t4h44e],cwathioinlestahnedcantiions and an 4444 usediaornesuhsoewdnarineFshigouwren2i.nEFxpigeurirmee2n.tEalxcpoenrcimenetrnattaiolncosnucsedntirnathioenwsourskeodfZinhtahoeetwaol.r[k23o]f Zhao e are of 0.5 (g/L) Li+ aqueous pha+se and an organic phase containing 0.1 (mol/L) of IL diluted al. [23] are of 0.5 (g/L) Li aqueous phase and an organic phase containing 0.1 (mol/L) o in methylbenzene at 298 K. Figure 2. Cont. (d) 5 y d y d = n ® d n d ]PDF Image | Ionic Liquids for the Selective Solvent Extraction of Lithium
PDF Search Title:
Ionic Liquids for the Selective Solvent Extraction of LithiumOriginal File Name Searched:
minerals-12-00190-v3.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |