
PDF Publication Title:
Text from PDF Page: 006
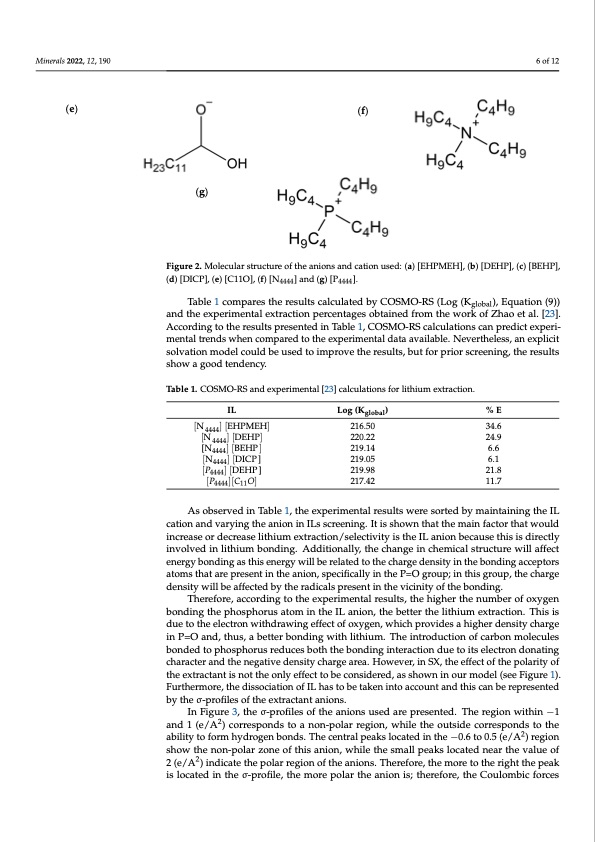
Minerals 2021, 11, x Minerals 2022, 12, 190 6 of 12 6 o (e) (f) (g) Figure 2. Molecular structure of the anions and cation used: (a) [EHPMEH], (b) [DEHP], (c) [BEHP], (d) [DICP], (e) [C11O], Figure 2. Molecular structure of the anions and cation used: (a) [EHPMEH], (b) [DEHP], (c) [BEHP], (f) [N4444] and (g) [P4444]. (d) [DICP], (e) [C11O], (f) [N4444] and (g) [P4444]. Table 1Tcaobmlep1arceosmthpearesutlthsecraelcsulatstecdablcyuClaOteSdMbOy-RCSO(SLMogO(-KRS (Lo),gE(qKugalotbioal)n, (E9q))uation ( global and the experimental extraction percentages obtained from the work of Zhao et al. [23]. and the experimental extraction percentages obtained from the work of Zhao et al. [2 According to the results presented in Table 1, COSMO-RS calculations can predict experi- According to the results presented in Table 1, COSMO-RS calculations can predict exp mental trends when compared to the experimental data available. Nevertheless, an explicit imental trends when compared to the experimental data available. Nevertheless, an solvation model could be used to improve the results, but for prior screening, the results plicit solvation model could be used to improve the results, but for prior screening, t show a good tendency. results show a good tendency. Table 1. COSMO-RS and experimental [23] calculations for lithium extraction. Table 1. COSMO-RS and experimental [23] calculations for lithium extraction. IL IL [N ] [EHPMEH] 4444[N4444] [EHPMEH] Log (Kglobal) %E [N4444] [DEHP] [N ] [DEHP] 216.50 34.6 [N4444] [BE44H44P] [N4444 ][N[DICP]] [BEHP] 220.22 24.9 220.22 24.9 4444 219.05 219.14 219.98 217.42 219.98 6.1 6.6 21.8 11.7 [P ] [DEHP] 4444 [N4444] [DICP] 219.05 6.1 21.8 [P4444][C11O] [𝑃4444] [DEHP] cation and varying the anion in ILs screening. It is shown that the main factor that would Log (Kglobal) %E 216.50 34.6 219.14 6.6 [ 𝑃4 4 4 4 ] [ 𝐶 𝑂 ] As observed in Table 1, the experimental results were sorted by maintaining the IL 2 1 7 . 4 2 1 1 . 7 As observed in Table 1, the experimental results were sorted by maintaining the increase or decrease lithium extraction/selectivity is the IL anion because this is directly cation and varying the anion in ILs screening. It is shown that the main factor that wo involved in lithium bonding. Additionally, the change in chemical structure will affect increase or decrease lithium extraction/selectivity is the IL anion because this is direc energy bonding as this energy will be related to the charge density in the bonding acceptors involved in lithium bonding. Additionally, the change in chemical structure will aff atoms that are present in the anion, specifically in the P=O group; in this group, the charge energy bonding as this energy will be related to the charge density in the bonding acc density will be affected by the radicals present in the vicinity of the bonding. Thtoersefaotroem, asccthoradtianrgetportehsenetxpinertihmeeanntaiolnre,supletsc,iftihcealhlyigihnetrhteheP=nOumgbroerupof; oinxythgiesngroup, t bondincghathrgeepdheonspsihtyorwusillatboemafifnecttheedIbLyanthioenr,atdhiecablestpterrestheentlitnhituhme veixctirnaictytionf.thTehibsoinsding. due to the eTlehcetroenfowreit,hadcrcaowrdininggeftfoecthoef oexypgeerinm, wenhtiaclhrpersouvlitds,esthaehhigighhererdethnseitnyucmhabregre of oxyg in P=O and, thus, a better bonding with lithium. The introduction of carbon molecules bonding the phosphorus atom in the IL anion, the better the lithium extraction. This is d bonded to phosphorus reduces both the bonding interaction due to its electron donating to the electron withdrawing effect of oxygen, which provides a higher density charge character and the negative density charge area. However, in SX, the effect of the polarity of P=O and, thus, a better bonding with lithium. The introduction of carbon molecu the extractant is not the only effect to be considered, as shown in our model (see Figure 1). bonded to phosphorus reduces both the bonding interaction due to its electron donati Furthermore, the dissociation of IL has to be taken into account and this can be represented character and the negative density charge area. However, in SX, the effect of the polar by the σ-profiles of the extractant anions. of the extractant is not the only effect to be considered, as shown in our model (see Fig In Figure 3, the σ-profiles of the anions used are presented. The region within −1 1). Furthermore, the dissociation of IL has to be taken into account and this can be rep and 1 (e/A2) corresponds to a non-polar region, while the outside corresponds to the sented by the σ-profiles of the extractant anions. 2 ability to form hydrogen bonds. The central peaks located in the −0.6 to 0.5 (e/A ) region In Figure 3, the σ-profiles of the anions used are presented. The region within −1 a show the non-polar zone of this anion, while the small peaks located near the value of 1 (e/A2) corresponds to a non-polar region, while the outside corresponds to the ability 2 (e/A2) indicate the polar region of the anions. Therefore, the more to the right the peak form hydrogen bonds. The central peaks located in the −0.6 to 0.5 (e/A2) region show t is located in the σ-profile, the more polar the anion is; therefore, the Coulombic forces non-polar zone of this anion, while the small peaks located near the value of 2 (e/A2) in cate the polar region of the anions. Therefore, the more to the right the peak is located the σ-profile, the more polar the anion is; therefore, the Coulombic forces between t f 9 e e u t e e l i u r n dPDF Image | Ionic Liquids for the Selective Solvent Extraction of Lithium
PDF Search Title:
Ionic Liquids for the Selective Solvent Extraction of LithiumOriginal File Name Searched:
minerals-12-00190-v3.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |