
PDF Publication Title:
Text from PDF Page: 007
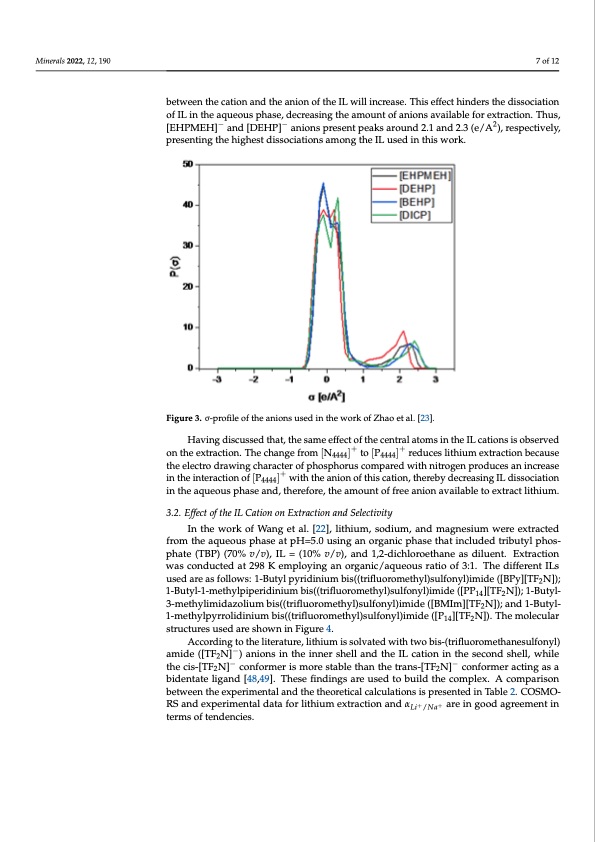
Minerals 2021, 11, x Minerals 2022, 12, 190 7 of 13 7 of 12 (a) (b) between the experimental and the theoretical calculations is presented in Table 2. COSMO- RS and experimental data for lithium extraction and αLi+/Na+ are in good agreement in terms of tendencies. cation and the anion of the IL will increase. This effect hinders the dissociation of IL in the aqueous phase, decreasing the amount of anions available for extraction. Thus, [EHP- MEH]- and [DEHP]- anions present peaks around 2.1 and 2.3 (e/A2), respectively, present- between the cation and the anion of the IL will increase. This effect hinders the dissociation ing the highest dissociations among the IL used in this work. of IL in the aqueous phase, decreasing the amount of anions available for extraction. Thus, [EHPMEH]− and [DEHP]− anions present peaks around 2.1 and 2.3 (e/A2), respectively, presenting the highest dissociations among the IL used in this work. Figure 3. σ-profile of the anions used in the work of Zhao et al. [23]. Figure 3. σ-profile of the anions used in the work of Zhao et al. [23]. Having discussed that, the same effect of the central atoms in the IL cations is observed on the extraction. The change from [N ]+ to [P ]+ reduces lithium extraction because Having discussed that, the same effect o44f4t4he centr4a4l44atoms in the IL cations is ob- served on the extraction. The change from [𝑁 ] to [𝑃 ] reduces lithium extrac- the electro drawing character of phosphorus compared with nitrogen produces an increase 3.2. Effect of the IL Cation on Extraction and Selectivity + with the anion of this cation, thereby decreasing IL dissociation in the interaction of [P ] tion because the electro draw4i4n44g character of phosphorus compared with nitrogen pro- in the aqueous phase and, therefore, the amount of free anion available to extract lithium. duces an increase in the interaction of [𝑃] with the anion of this cation, thereby de- creasing IL dissociation in the aqueous phase and, therefore, the amount of free anion 3.2. Effect of the IL Cation on Extraction and Selectivity available to extract lithium. In the work of Wang et al. [22], lithium, sodium, and magnesium were extracted from the aqueous phase at pH=5.0 using an organic phase that included tributyl phos- phate (TBP) (70% v/v), IL = (10% v/v), and 1,2-dichloroethane as diluent. Extraction In the work of Wang et al. [22], lithium, sodium, and magnesium were extracted from was conducted at 298 K employing an organic/aqueous ratio of 3:1. The different ILs the aqueous phase at pH=5.0 using an organic phase that included tributyl phosphate used are as follows: 1-Butyl pyridinium bis((trifluoromethyl)sulfonyl)imide ([BPy][TF2N]); (TBP) (70% v/v), IL = (10% v/v), and 1,2-dichloroethane as diluent. Extraction was con- 1-Butyl-1-methylpiperidinium bis((trifluoromethyl)sulfonyl)imide ([PP14][TF2N]); 1-Butyl- ducted at 298 K employing an organic/aqueous ratio of 3:1. The different ILs used are as 3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide ([BMIm][TF2N]); and 1-Butyl- follows: 1-Butyl pyridinium bis((trifluoromethyl)sulfonyl)imide ([BPy][TF2N]); 1-Butyl-1- 1-methylpyrrolidinium bis((trifluoromethyl)sulfonyl)imide ([P14][TF2N]). The molecular methylpiperidinium bis((trifluoromethyl)sulfonyl)imide ([PP14][TF2N]); 1-Butyl-3-me- structures used are shown in Figure 4. thylimidazolium bis((trifluoromethyl)sulfonyl)imide ([BMIm][T F2N ]); and 1-Butyl-1- According to the literature, lithium is solvated with two bis-(trifluoromethanesulfonyl) methylpyrrolidinium bis((trifluoromethyl)sulfonyl)imide ([ P ][T F N ]). The molecular − 142 amide ([TF2N] ) anions in the inner shell and the IL cation in the second shell, while structures used are sho−wn in Figure 4. − the cis-[TF2N] conformer is more stable than the trans-[TF2N] conformer acting as a bidentate ligand [48,49]. These findings are used to build the complex. A comparisonPDF Image | Ionic Liquids for the Selective Solvent Extraction of Lithium
PDF Search Title:
Ionic Liquids for the Selective Solvent Extraction of LithiumOriginal File Name Searched:
minerals-12-00190-v3.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |