
PDF Publication Title:
Text from PDF Page: 008
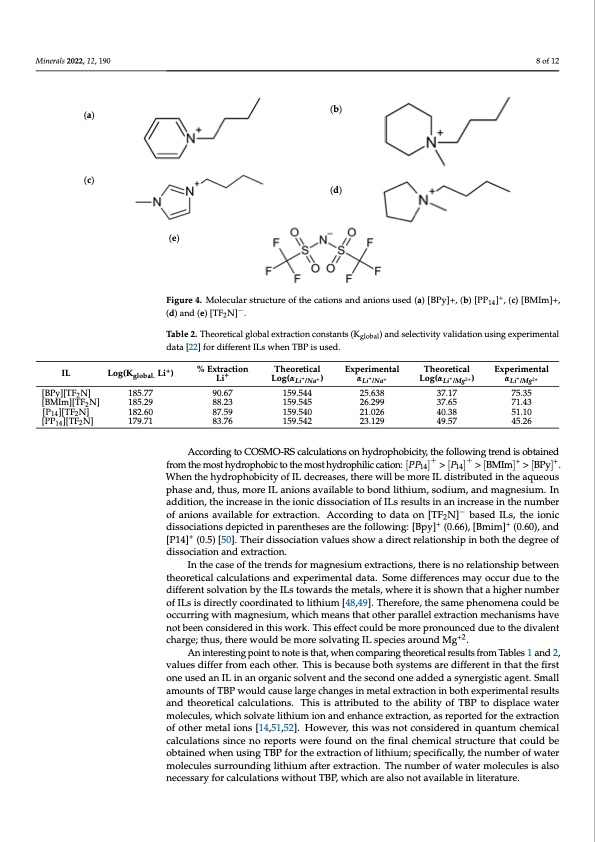
the aqueous phase at pH=5.0 using an organic phase that included tributyl phosphate 235 (TBP) (70% v/v), IL = (10% v/v), and 1,2-dichloroethane as diluent. Extraction was con- 236 ducted at 298 K employing an organic/aqueous ratio of 3:1. The different ILs used are as 237 follows: 1-Butyl pyridinium bis((trifluoromethyl)sulfonyl)imide ([BPy][TF2 N]); 1-Butyl-1- 238 methylpiperidinium bis((trifluoromethyl)sulfonyl)imide ([P P14 ][T F2 N ]); 1-Butyl-3-me- 239 thylimidazolium bis((trifluoromethyl)sulfonyl)imide ([BMIm][T F2 N ]); and methylpyrrolidinium bis((trifluoromethyl)sulfonyl)imide ([ P14 ][T F2 N ]). The (b) (d) Minerals 2022, 12, 190 (a) Minerals 2021, 11, x (c) IL Log(K Li+) %deEnxttartaectliognand[48T,4h9e]o.rTehtiecsaelfindinEgxpsearriemuensetadltobuiTldhetohretcicoamlplex.EAxpceormimpeanritsaolnbe- 8 of 12 1-Butyl-1- 240 molecular 241 242 8 of 13 (e) Figure 4. Molecular structure of the cations and anions used (a) [BPy]+, (b) [PP +]+, (c) [BMIm]+, (d) Figure 4. Molecular structure of the cations and anions used (a) [BPy]+, (b) [PP141]4 , (c) [BMIm]+, and (e) [TF N]−. (d) and (e) [TF2N]−2. According to the literature, lithium is solvated with two bis-(trifluoromethanesul- Table 2. Theoretical global extraction constants (Kglobal) and selectivity validation using experimental fonyl)amide ([TF N]−) anions in the inner shell and the IL cation in the second shell, while data[22]fordifferentILsw2henTBPisused. the cis-[TF2N]− conformer is more stable than the trans-[TF2N]− conformer acting as a bi- global. 185.77 185.29 182.60 179.71 Li+ tween the experimentaLlian/Nda the theoretLiica/Nlacalculations is Lpire/Msegnted in TabLlie/M2.gCOSMO- Log(α + +) α + + Log(α + 2+) α + 2+ [BPy][TF N] 2 [BMIm][TF N] 2 [P14 ][TF2 N] [PP14 ][TF2 N] RS 9a0n.d67experimenta1l5d9a.5t4a4for lithium 2e5x.t6r3a8ction and 𝛼 37.17 / are in good7a5.g3r5eement in 71.43 51.10 88.23 159.545 26.299 37.65 terms of tendencies. 87.59 159.540 21.026 40.38 According to COSMO-RS calculations on hydrophobicity, the following trend is ob- 83.76 159.542 23.129 49.57 45.26 t a i n e d f r o m t h e m o s t h y d r o p h o b i c t o t h e m o s t h y d r o p h i l i c c a t i o n : [ 𝑃 𝑃 ] > [𝑃] > [BMIm]+ > [BPy]+. When the hydrophobicity of IL decreases, there will be more IL According to COSMO-RS calculations on hydrophobicity, the following trend is obtained distributed in the aqueous phase and, thus, more IL anions available to bond lithium, so- fromthemosthydrophobictothemosthydrophiliccation:[PP ]+>[P ]+>[BMIm]+>[BPy]+. dium, and magnesium. In addition, the increase in th14e ionic d14issociation of ILs results in When the hydrophobicity of IL decreases, there will be more IL distributed in the aqueous - an increase in the number of anions available for extraction. According to data on [TF2N] phase and, thus, more IL anions available to bond lithium, sodium, and magnesium+. In based ILs, the ionic dissociations depicted in parentheses are the following: [Bpy] (0.66), ++ addition[B,mthiemi]nc(0re.6a0s)e,aindth[eP1io4n]ic(0d.5i)ss[o50c]i.atTihoenirodfiIsLssocrieastuioltnsvinalaunesinshcorewasaedinirethcterneluamtiobnesrhip of anioninsbaovthaitlhabeldeefgorreeeoxftrdaicstsiocnia.tiAonccaonrdeinxgtratcotidona.ta on [TF2N]− based ILs, the ionic ++ dissociationIsndtehpeictaesdeoinftphaerternetnhdesefosramreatghnesfoiullmowexintrga:c[tiBopnys,]th(e0r.e66is),n[Bomreilmat]ion(s0h.6ip0)b,eatnwdeen [P14]+ (t0h.e5o)r[e5t0ic].alThcaelicrudlaistisoncsiaatinodnevxapleureismsehnotawl daadtair.eScotmreeladtiioffnesrhenipceisnmboatyhothcceudredgureetofthe dissociadtifofneraentdseoxlvtraaticotniobny. the ILs towards the metals, where it is shown that a higher number InothfeILcsaissedoifretchtelytrceonodrdsifnoartemdatgonlietshiiume[4x8tr,4a9c]t.ioTnhse,rtehfoere,itshneosarmeleatpiohnenshomipebneatwcoeuelnd be occurring with magnesium, which means that other parallel extraction mechanisms have theoretical calculations and experimental data. Some differences may occur due to the not been considered in this work. This effect could be more pronounced due to the diva- different solvation by the ILs towards the metals, where it is shown that a higher number lent charge; thus, there would be more solvating IL species around Mg+2. of ILs is directly coordinated to lithium [48,49]. Therefore, the same phenomena could be occurring with magnesium, which means that other parallel extraction mechanisms have Table 2. Theoretical global extraction constants (Kglobal) and selectivity validation using experimental data [22] for different not been considered in this work. This effect could be more pronounced due to the divalent ILs when TBP is used. +2 charge; thus, there would be more solvating IL species around Mg . IL An intere%stinEgxtpraocintitotno noteTihsethoaret,tiwcahlen comEpxpareirnigmtehnetoarleticaTl rheesourletstifcraolm TaEbxlepser1imanednt2a,l [BPy][TF2N] [BMIm][TF2N] [P14][TF2N] [PP14][TF2N] 𝑳𝒊 /𝑵𝒂 𝑳𝒊 /𝑵𝒂 𝑳𝒊 /𝑴𝒈 𝑳𝒊 /𝑴𝒈 one used an IL in an organic solvent and the second one added a synergistic agent. Small Log(Kglobal. Li+) valuesdifferfromLeiachotheLr.oTgh(𝜶isisbec)ausebo𝜶thsystemsaLroegd(𝜶ifferen𝟐ti)ntha𝜶tthefi𝟐rst + 185.77 90.67 159.544 25.638 37.17 75.35 amounts of TBP would cause large changes in metal extraction in both experimental results 185.29 88.23 159.545 26.299 37.65 71.43 and theoretical calculations. This is attributed to the ability of TBP to displace water 182.60 87.59 159.540 21.026 40.38 51.10 molecules, which solvate lithium ion and enhance extraction, as reported for the extraction 179.71 83.76 159.542 23.129 49.57 45.26 of other metal ions [14,51,52]. However, this was not considered in quantum chemical calculations since no reports were found on the final chemical structure that could be obtained when using TBP for the extraction of lithium; specifically, the number of water molecules surrounding lithium after extraction. The number of water molecules is also necessary for calculations without TBP, which are also not available in literature.PDF Image | Ionic Liquids for the Selective Solvent Extraction of Lithium
PDF Search Title:
Ionic Liquids for the Selective Solvent Extraction of LithiumOriginal File Name Searched:
minerals-12-00190-v3.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |