
PDF Publication Title:
Text from PDF Page: 009
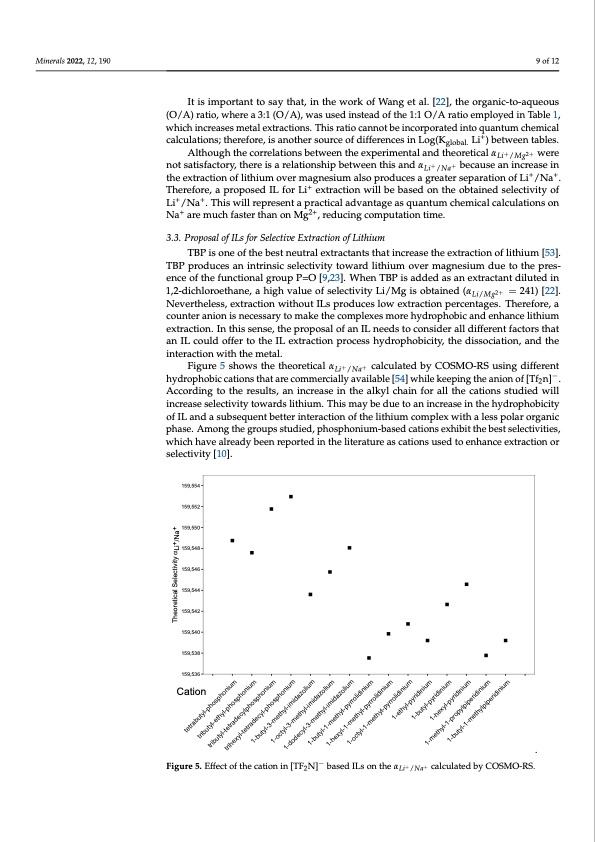
Minerals 2022, 12, 190 9 of 12 Minerals 2021, 11, x It is important to say that, in the work of Wang et al. [22], the organic-to-aqueous (O/A) ratio, where a 3:1 (O/A), was used instead of the 1:1 O/A ratio employed in Table 1, which increases metal extractions. This ratio cannot be incorporated into quantum chemical calculations; therefore, is another source of differences in Log(Kglobal. Li+) between tables. AlthoughthecorrelationsbetweentheexperimentalandtheoreticalαLi+/Mg2+ were notsatisfactory,thereisarelationshipbetweenthisandαLi+/Na+ becauseanincreasein the extraction of lithium over magnesium also produces a greater separation of Li+/Na+. Therefore, a proposed IL for Li+ extraction will be based on the obtained selectivity of Li+/Na+. This will represent a practical advantage as quantum chemical calculations on Na+ are much faster than on Mg2+, reducing computation time. 3.3. Proposal of ILs for Selective Extraction of Lithium TBP is one of the best neutral extractants that increase the extraction of lithium [53]. TBP produces an intrinsic selectivity toward lithium over magnesium due to the pres- ence of the functional group P=O [9,23]. When TBP is added as an extractant diluted in 1,2-dichloroethane, a high value of selectivity Li/Mg is obtained (αLi/Mg2+ = 241) [22]. Nevertheless, extraction without ILs produces low extraction percentages. Therefore, a counter anion is necessary to make the complexes more hydrophobic and enhance lithium extraction. In this sense, the proposal of an IL needs to consider all different factors that an IL could offer to the IL extraction process hydrophobicity, the dissociation, and the interaction with the metal. Figure 5 shows the theoretical αLi+/Na+ calculated by COSMO-RS using different hydrophobic cations that are commercially available [54] while keeping the anion of [Tf2n]−. According to the results, an increase in the alkyl chain for all the cations studied will increase selectivity towards lithium. This may be due to an increase in the hydrophobicity of IL and a subsequent better interaction of the lithium complex with a less polar organic phase. Among the groups studied, phosphonium-based cations exhibit the best selectivities, which have already been reported in the literature as cations used to enhance extra10ctoifo1n3 or selectivity [10]. 159,554 159,552 159,550 159,548 159,546 159,544 159,542 159,540 159,538 159,536 Cation − A theoretical model was developed to calculate selectivities in the SX of lithium over sodium and magnesium from brines using COSMO-RS. Figure 5. Effect of the cation in [TF N]− based ILs on the α + + calculated by COSMO-RS. Figure 5. Effect of the cation in [TF2N] based ILs on the 𝛼 Li /Nacalculated by COSMO-RS. 4. Conclusions 2 / y The developed model was first validated using experimental data of lithium extrac- . Theoretical Selectivity αLi+/Na+ tetrabutyl-phosphonium tributyl-ethyl-phosphonium tributyl-tetradecylphosphonium trihexyl-tetradecyl-phosphonium 1-butyl-3-methyl-imidazolium 1-octyl-3-methyl-imidazolium 1-dodecyl-3-methyl-imidazolium 1-butyl-1-methyl-pyrrolidinium 1-hexyl-1-methyl-pyrrolidinium 1-octyl-1-methyl-pyrrolidinium 1-ethyl-pyridinium 1-butyl-pyridinium 1-hexyl-pyridinium 1-methyl-1-propylpiperidinium 1-butyl-1-methylpiperidinium tion from brines. Thus, theoretical calculations showed acceptable agreements with exper-PDF Image | Ionic Liquids for the Selective Solvent Extraction of Lithium
PDF Search Title:
Ionic Liquids for the Selective Solvent Extraction of LithiumOriginal File Name Searched:
minerals-12-00190-v3.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |