
PDF Publication Title:
Text from PDF Page: 006
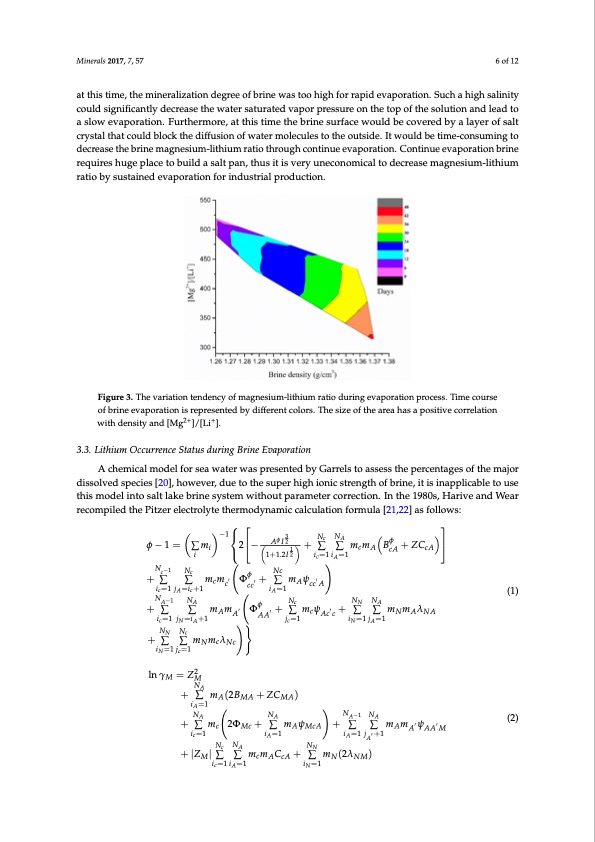
1.3634 0 100 0 1.3648 0 100 0 1.3654 0 100 0 1.3668 0 100 0 1.3891 1 99 0 Minerals 2017, 7, 57 1.3692 0 100 0 6 of 12 The relationship of magnesium-lithium ratio between brine densities during the evaporation at this time, the mineralization degree of brine was too high for rapid evaporation. Such a high salinity process is given in Figure 3. It shows that the Magnesium-lithium ratio does not changed much in could significantly decrease the water saturated vapor pressure on the top of the solution and lead to early evaporation stage (depicted in magenta and violet colors), but a larger change appears when a slow evaporation. Furthermore, at this time the brine surface would be covered by a layer of salt the brine density value is more than 1.285 g/cm3 (depicted in blue and green colors). The magnesium- crystal that could block the diffusion of water molecules to the outside. It would be time-consuming to lithium ratio decreases sharply in the later stage of evaporation, especially when brine density is more decrease the brine magnesium-lithium ratio through continue evaporation. Continue evaporation brine than 1.345 g/cm3. Brine magnesium-lithium ratio decreases almost to half of initial value when the requires huge place to build a salt pan, thus it is very uneconomical to decrease magnesium-lithium brine density is around 1.372 g/cm3 (depicted in red). ratio by sustained evaporation for industrial production. Figure 3. The variation tendency of magnesium-lithium ratio during evaporation process. Time course Figure 3. The variation tendency of magnesium-lithium ratio during evaporation process. Time of brine evaporation is represented by different colors. The size of the area has a positive correlation course of brine evaporation is represented by different colors. The size of the area has a positive with density and [Mg2+]/[Li+]. 2+ + correlationwithdensityand[Mg ]/[Li]. 3.3. Lithium Occurrence Status during Brine Evaporation A chemical model for sea water was presented by Garrels to assess the percentages of the major dissolved species [20], however, due to the super high ionic strength of brine, it is inapplicable to use this model into salt lake brine system without parameter correction. In the 1980s, Harive and Wear recompiled the Pitzer electrolyte thermodynamic calculation formula [21,22] as follows: −1AφI23 NcNA φ φ−1= ∑mi 2− 1+∑ ∑mcmA BcA+ZCcA i 1+1.2I 2 ic=1 iA=1 Nc−1Nc Nc φ ic=1 j =ic+1 i =1 A A NA−1 NA φ Nc +∑ ∑mAmA′ ΦAA′ ic=1 jN=iA+1 +∑mψ′ +∑ ∑mcmc′ Φcc′ AccA (1) (2) NN Nc + ∑ ∑ mNmcλNc iN=1 jc=1 lnγM =Z2M NA + ∑ mA(2BMA+ZCMA) +∑mψ′ NN NA +∑∑mmλ iN=1 jA=1 jc=1 cAcc NANA iA=1 NANA NA−1NA + ∑ mc 2ΦMc+ ∑ mAψMcA + ∑ ∑ mAmA′ψAA′M ic=1 iA=1 iA=1 jA′ +1 Nc NA NN + |ZM| ∑ ∑ mcmACcA + ∑ mN(2λNM) ic=1iA=1 iN=1PDF Image | Lithium during Brine Evaporation and KCl Production Plants
PDF Search Title:
Lithium during Brine Evaporation and KCl Production PlantsOriginal File Name Searched:
minerals-07-00057-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |