
PDF Publication Title:
Text from PDF Page: 010
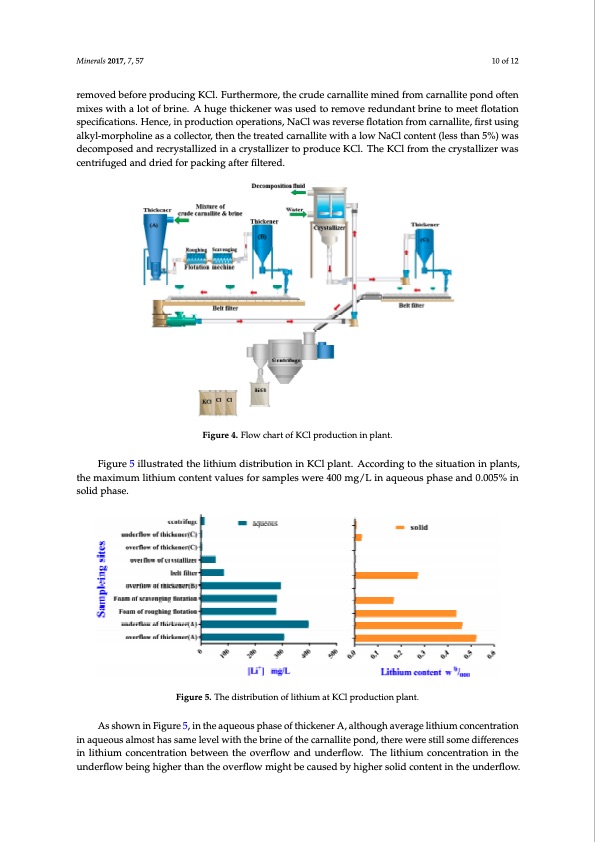
Minerals 2017, 7, 57 10 of 12 removed before producing KCl. Furthermore, the crude carnallite mined from carnallite pond often mixes with a lot of brine. A huge thickener was used to remove redundant brine to meet flotation sMpMienicnerieafirlasclsa220t0i1o177,n,7s7,.,5H577ence, in production operations, NaCl was reverse flotation from carnallite, first1u10s0oiofnf1g122 alkyl-morpholine as a collector, then the treated carnallite with a low NaCl content (less than 5%) was waassddeeccoomppoosseeddaannddrreeccrryysstatallilzizeeddininaaccrryysstatallilzizeerrtotopprroodduucceeKKCCl.l.TThheeKKCCl lfrfroomththeeccrryysstatallilzizeerr decomposed and recrystallized in a crystallizer to produce KCl. The KCl from the crystallizer was waasscceenntrtrifiufuggeeddaannddddrrieieddfoforrppaacckkininggaaftfeterrfiflitleterreedd. . centrifuged and dried for packing after filtered. FFigiguurere4.4F. Flolowwchcharatrtoof fKKCCl pl prordoduuctcitoinonininpplalnatn.t. Figure 4. Flow chart of KCl production in plant. FFigiguurree55ililulusstrtraateteddththeeliltihthiuiumddisistrtribibuutitoionnininKKCCl lpplalannt.t.Acccoorrddininggtotoththeessitiutuaatitoionnininpplalanntsts, , Figure 5 illustrated the lithium distribution in KCl plant. According to the situation in plants, ththeemaaxximimuumliltihthiuiumccoonntetennttvvaalulueessfoforrssaampplelessweerree44000mgg/L/Lininaaqquueeoouusspphhaasseeaanndd00.0.0055%inin the maximum lithium content values for samples were 400 mg/L in aqueous phase and 0.005% in ssoolildidpphhaassee. . solid phase. Figure 5. The distribution of lithium at KCl production plant. FFigiguurere55..TThheeddisistrtirbibuutitoionnooffliltihthiuiumaattKKCCllpprorodduucctitoionnpplalannt.t. Asshsshohwoownwnin iFniinguFFirgiegu5ur,reien5t5h, ,einianquthteheoeuasaqpquhueaeosoueusosfptphhiacaskseenoeofrfAtht,hiaciclktkheenoneuergrhAa,v,eaarlatlhtghoeouluigtghhiuamavveceroranaggceenltilrtihathtiiuioumnm icncooannqccueenenotrturasatitaoilonmnioninstaaqhquauseeosoauumssaeallmlemvoeoslstwthhaitashsstsahamemeberlielneveveeol lfwthitiehthctahthrenebablrlrinitneepooffnthtdhe,etchcaearrnenawallieltiretepspotoinlnldsd,o,tmhtheerdreeiwfwferererenescsteitlsill isnsoomlmiteheidudimfifefceroreenncceensstrinaintiloiltnihthibuieumtmwecceoonnctcehenentrtoraavtiteoironflnobwbeetwtawnedeenunnthtdheerfloovovewer.rflfolTowhwe alanitndhdiuumnnddceoerrfnlfocloewn.t.rTaTthiheoenlilitinhthituihumem ucconondncecerenflntortwraatitboioeninigninhthitgheheeuurnntdhdeaernrflfotlohwe obbveeinirnflggohwhigigmhheiegrrhtththbaanenctahthueeseoodvveberyrflfohlowiwghmemrigisghohtlitdbbeceocncaatueusnseteddinbbtyhyehhiugignhhdeerrsflsolwildid. ccoonntetennttininththeeuunnddeerrflfolow. .TThheessuurrvveeyysshhooweeddththaattssoolildidliltihthiuiumccoonntetennttooffththeessccaavveennggininggflfolotatatitoionn fofoaampphhaasseewoouuldldhhaavveeaannoobbvvioiouussddeeccrreeaasseeccoomppaarreeddwitihthththeerroouugghhininggflfolotatatitoionnfofoaampphhaassee. . Hooweevveerr, ,ththeerreeaassoonnssfoforrththisispphheennoomeennoonnaarreennoottvveerryyccleleaarraannddththeerreeisisliltitltelerreesseeaarrcchhrreeppoorrteteddoonn iti.t.Aftfeterrththeefifrirssttddee-b-brrininee, ,ththeesshhaarrppddeeccrreeaasseeininththeeccoonntetennttooffliltihthiuiumininbbooththththeeaaqquueeoouussaannddssoolildid p ph ha as se es s c co ou ul dl d b be e d du ue e t ot o d dr ri pi p w wa as sh hi ni ng g: : t ot o m me e e et t p pu ur ri t i yt y s sp pe ec ci f i i f ci ca at i t oi on ns s o of f f i f ni na al l KKCCl , l , t ht he e e ea as sy y s so ol ul ub bl el e i oi on ns s aabbssoorrbbeeddoonnththeessuurrfafacceeooffccaarrnnaalliltiete, ,ssuucchhaassliltihthiuiumaannddmaaggnneessiuium, ,aarreeaalwlwaayyssrreemoovveeddbbyyddrripipPDF Image | Lithium during Brine Evaporation and KCl Production Plants
PDF Search Title:
Lithium during Brine Evaporation and KCl Production PlantsOriginal File Name Searched:
minerals-07-00057-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |