
PDF Publication Title:
Text from PDF Page: 006
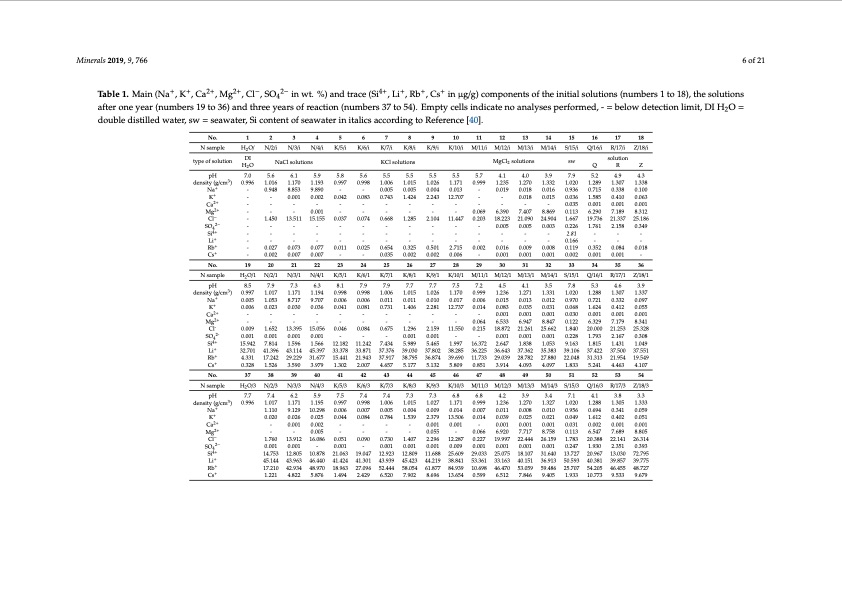
Minerals 2019, 9, 766 6 of 21 Table 1. Main (Na+, K+, Ca2+, Mg2+, Cl−, SO42− in wt. %) and trace (Si4+, Li+, Rb+, Cs+ in μg/g) components of the initial solutions (numbers 1 to 18), the solutions after one year (numbers 19 to 36) and three years of reaction (numbers 37 to 54). Empty cells indicate no analyses performed, - = below detection limit, DI H2O = double distilled water, sw = seawater, Si content of seawater in italics according to Reference [40]. No. N sample type of solution 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 H2O/ N/2/i N/3/i N/4/i K/5/i K/6/i K/7/i K/8/i K/9/i K/10/i M/11/i M/12/i M/13/i M/14/i S/15/i Q/16/i R/17/i Z/18/i DI NaCl solutions KCl solutions MgCl2 solutions sw solution H2O QRZ pH 7.0 5.6 6.1 5.9 5.8 5.6 5.5 5.5 5.5 5.5 5.7 4.1 4.0 3.9 7.9 5.2 4.9 4.3 density (g/cm3) Na+ K+ Ca2+ Mg2+ Cl− SO4 2− Si4+ Li+ Rb+ Cs+ No. N sample pH 8.5 7.9 7.3 6.3 8.1 7.9 7.9 7.7 7.7 7.5 7.2 4.5 4.1 3.5 7.8 5.3 4.6 3.9 0.996 1.016 1.170 - 0.948 8.853 - - 0.001 1.193 0.997 0.998 9.890 -- 0.002 0.042 0.083 1.006 1.015 0.005 0.005 0.743 1.424 1.026 0.004 2.243 1.171 0.999 1.235 1.270 0.013 - 0.019 0.018 12.707 - - 0.018 1.332 0.016 0.015 1.020 1.289 0.936 0.715 0.036 1.585 0.035 0.001 0.113 6.290 1.667 19.736 0.226 1.761 1.307 1.338 0.338 0.100 0.410 0.063 0.001 0.001 7.189 8.312 21.337 25.186 2.158 0.349 - - - - - - - - ------------- - - 0.001 - - - - 0.668 1.285 - - - - 2.104 11.447 - - 0.069 6.390 7.407 8.869 0.203 18.223 21.090 24.904 - 0.005 0.005 0.003 1.450 - 13.511 15.155 0.037 0.074 -- - - - - - - - - - - - - - - - 2.81 - - - ------------- 0.166 --- 0.027 0.073 0.077 0.011 0.025 0.654 0.325 0.501 2.715 0.002 0.016 0.009 0.008 0.119 0.352 0.084 0.018 0.002 0.007 0.007 -- 0.035 0.002 0.002 0.006 - 0.001 0.001 0.001 0.002 0.001 0.001 - 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 H2O/1 N/2/1 N/3/1 N/4/1 K/5/1 K/6/1 K/7/1 K/8/1 K/9/1 K/10/1 M/11/1 M/12/1 M/13/1 M/14/1 S/15/1 Q/16/1 R/17/1 Z/18/1 density (g/cm3) Na+ K+ Ca2+ Mg2+ Cl- SO42- Si4+ Li+ Rb+ Cs+ No. N sample pH 7.7 7.4 6.2 5.9 7.5 7.4 7.4 7.3 7.3 6.8 6.8 4.2 3.9 3.4 7.1 4.1 3.8 3.3 0.997 1.017 1.171 0.005 1.053 8.717 0.006 0.023 0.030 1.194 9.707 0.036 0.998 0.006 0.041 - - 0.046 0.998 0.006 0.081 - - 0.084 1.006 0.011 0.731 - - 0.675 1.015 1.026 1.170 0.999 0.011 0.010 0.017 0.006 1.406 2.281 12.737 0.014 - - - - - - - 0.064 1.296 2.159 11.550 0.215 1.236 1.271 0.015 0.013 0.083 0.035 0.001 0.001 6.533 6.947 1.331 0.012 0.031 0.001 8.847 1.020 0.970 0.068 0.030 0.122 1.840 0.228 9.163 38.285 36.225 36.643 37.362 35.383 39.106 39.690 11.733 29.039 28.782 27.880 22.048 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 - - -- -- 13.395 15.056 1.337 0.097 0.055 0.001 8.341 25.328 - - 0.009 1.652 0.001 0.001 15.942 7.814 18.872 21.261 25.662 - - 0.001 0.001 0.001 1.997 16.372 2.647 1.838 1.053 0.001 0.001 - - - 1.596 1.566 12.182 11.242 7.434 32.701 41.396 43.114 45.397 33.378 33.871 37.376 39.030 37.802 4.331 17.242 29.229 31.677 15.441 21.943 37.917 38.795 36.874 0.328 1.526 3.590 3.979 1.302 2.007 4.657 5.177 5.132 0.001 0.001 5.989 5.465 31.313 21.954 19.549 5.809 0.851 3.914 4.093 4.097 1.833 5.241 4.463 4.107 1.288 1.307 0.721 0.332 1.624 0.412 0.001 0.001 6.329 7.179 20.000 21.253 1.793 2.167 0.308 1.815 1.431 1.049 37.422 37.500 37.551 H2O/3 N/2/3 N/3/3 N/4/3 K/5/3 K/6/3 K/7/3 K/8/3 K/9/3 K/10/3 M/11/3 M/12/3 M/13/3 M/14/3 S/15/3 Q/16/3 R/17/3 Z/18/3 density (g/cm3) Na+ K+ Ca2+ Mg2+ Cl− SO4 2− Si4+ Li+ Rb+ Cs+ 0.996 1.017 1.171 1.110 9.129 0.020 0.026 1.760 13.912 16.086 0.051 0.090 0.730 1.407 0.001 0.001 - 0.001 - 0.001 0.001 1.195 0.997 0.998 10.298 0.006 0.007 0.025 0.044 0.084 1.006 1.015 0.005 0.004 0.784 1.539 1.027 0.009 2.379 0.001 0.055 2.296 0.001 1.171 0.999 1.236 0.014 0.007 0.011 13.506 0.014 0.039 0.001 - 0.001 - 0.066 6.920 12.287 0.227 19.997 0.009 0.001 0.001 25.609 29.033 25.075 38.841 53.361 33.163 84.939 10.698 46.470 1.270 1.327 0.008 0.010 0.025 0.021 0.001 0.001 7.717 8.758 22.444 26.159 0.001 0.001 1.020 0.956 0.049 0.031 0.113 1.783 0.247 1.288 1.305 0.694 0.341 1.612 0.402 0.002 0.001 6.547 7.689 20.388 22.141 1.930 2.351 20.967 13.030 40.381 39.857 54.205 46.455 1.333 0.059 0.051 0.001 8.805 26.314 0.393 72.795 39.775 48.727 0.002 - - - - - - 0.005 - - - - - 0.001 14.753 12.805 10.878 21.063 19.047 12.923 12.809 11.688 45.144 43.963 46.440 41.424 41.301 43.939 45.423 44.219 17.210 42.934 48.970 18.963 27.096 52.444 58.054 61.877 1.221 4.822 5.876 1.494 2.429 6.520 7.902 8.696 18.107 31.640 13.727 40.151 36.913 50.593 53.059 59.486 25.707 13.654 0.599 6.512 7.846 9.405 1.933 10.773 9.533 9.679PDF Image | Lithium Occurrences in Brines from Two German Salt Deposits
PDF Search Title:
Lithium Occurrences in Brines from Two German Salt DepositsOriginal File Name Searched:
minerals-09-00766-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |