
PDF Publication Title:
Text from PDF Page: 007
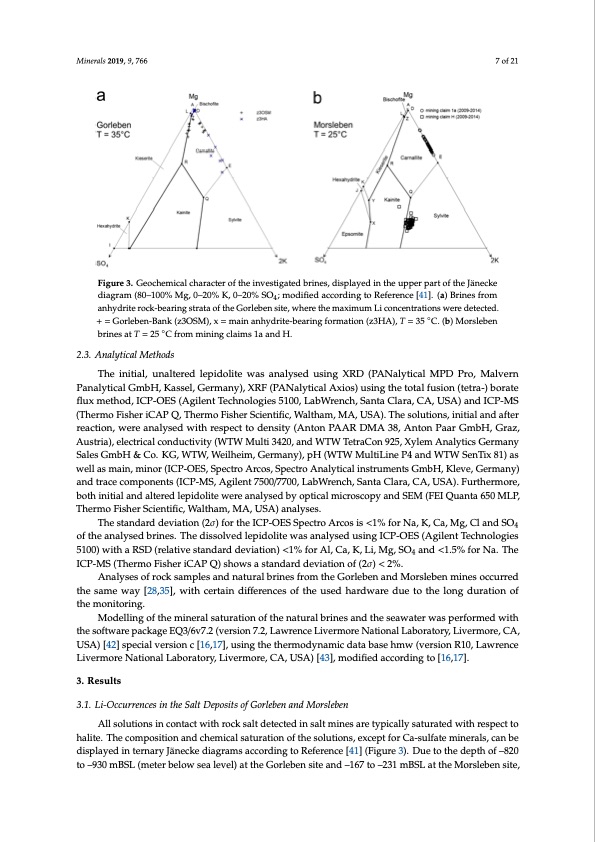
the same way [28,35], with certain differences of the used hardware due to the long duration of the monitoring. Modelling of the mineral saturation of the natural brines and the seawater was performed with the software package EQ3/6v7.2 (version 7.2, Lawrence Livermore National Laboratory, Livermore, CA, USA) [42] special version c [16,17], using the thermodynamic data base hmw (version R10, Minerals 2019, 9, 766 7 of 21 Lawrence Livermore National Laboratory, Livermore, CA, USA) [43], modified according to [16,17]. Figure 3. Geochemical character of the investigated brines, displayed in the upper part of the Jänecke diagram (80–100% Mg, 0–20% K, 0–20% SO4; modiffiied according to Reference [41]. (a) Brines from 4 anhydriterorocckk-b-beaearirninggstrsatrtatoaftohfetGheorGleobrelnebsietne,wsithee,rewthermeathxiemmumaxLimicuomnceLnitrcaotniocnesnwtraetrieodnestewcterde. +de=teGctoerdle.b+en=-GBaonrlkeb(ze3nO-BSaMn)k, x(z=3OmSaMin),axnh=ymdraiitne-abneahryidngritfeo-rbmeartionng (fzo3rHmAat)i,oTn=(z35HAC).,(Tb)=M3o5r°sCle.b(ebn) ◦ bMrionressleabteTn =br2in5esCatfrTom= 2m5i°nCinfgrocmlaimisn1inagacnldaiHm.s 1a and H. 2.3. Analytical Methods 3. Results The initial, unaltered lepidolite was analysed using XRD (PANalytical MPD Pro, Malvern 3.1. Li-Occurrences in the Salt Deposits of Gorleben and Morsleben Panalytical GmbH, Kassel, Germany), XRF (PANalytical Axios) using the total fusion (tetra-) borate flux method, ICP-OES (Agilent Technologies 5100, LabWrench, Santa Clara, CA, USA) and ICP-MS All solutions in contact with rock salt detected in salt mines are typically saturated with respect (Thermo Fisher iCAP Q, Thermo Fisher Scientific, Waltham, MA, USA). The solutions, initial and after to halite. The composition and chemical saturation of the solutions, except for Ca-sulfate minerals, reaction, were analysed with respect to density (Anton PAAR DMA 38, Anton Paar GmbH, Graz, can be displayed in ternary Jänecke diagrams according to Reference [41] (Figure 3). Due to the Austria), electrical conductivity (WTW Multi 3420, and WTW TetraCon 925, Xylem Analytics Germany depth of –820 to –930 mBSL (meter below sea level) at the Gorleben site and –167 to –231 mBSL at the Sales GmbH & Co. KG, WTW, Weilheim, Germany), pH (WTW MultiLine P4 and WTW SenTix 81) as well as main, minor (ICP-OES, Spectro Arcos, Spectro Analytical instruments GmbH, Kleve, Germany) and trace components (ICP-MS, Agilent 7500/7700, LabWrench, Santa Clara, CA, USA). Furthermore, both initial and altered lepidolite were analysed by optical microscopy and SEM (FEI Quanta 650 MLP, Thermo Fisher Scientific, Waltham, MA, USA) analyses. The standard deviation (2σ) for the ICP-OES Spectro Arcos is <1% for Na, K, Ca, Mg, Cl and SO4 of the analysed brines. The dissolved lepidolite was analysed using ICP-OES (Agilent Technologies 5100) with a RSD (relative standard deviation) <1% for Al, Ca, K, Li, Mg, SO4 and <1.5% for Na. The ICP-MS (Thermo Fisher iCAP Q) shows a standard deviation of (2σ) < 2%. Analyses of rock samples and natural brines from the Gorleben and Morsleben mines occurred the same way [28,35], with certain differences of the used hardware due to the long duration of the monitoring. Modelling of the mineral saturation of the natural brines and the seawater was performed with the software package EQ3/6v7.2 (version 7.2, Lawrence Livermore National Laboratory, Livermore, CA, USA) [42] special version c [16,17], using the thermodynamic data base hmw (version R10, Lawrence Livermore National Laboratory, Livermore, CA, USA) [43], modified according to [16,17]. 3. Results 3.1. Li-Occurrences in the Salt Deposits of Gorleben and Morsleben All solutions in contact with rock salt detected in salt mines are typically saturated with respect to halite. The composition and chemical saturation of the solutions, except for Ca-sulfate minerals, can be displayed in ternary Jänecke diagrams according to Reference [41] (Figure 3). Due to the depth of –820 to –930 mBSL (meter below sea level) at the Gorleben site and –167 to –231 mBSL at the Morsleben site, ◦PDF Image | Lithium Occurrences in Brines from Two German Salt Deposits
PDF Search Title:
Lithium Occurrences in Brines from Two German Salt DepositsOriginal File Name Searched:
minerals-09-00766-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |