
PDF Publication Title:
Text from PDF Page: 004
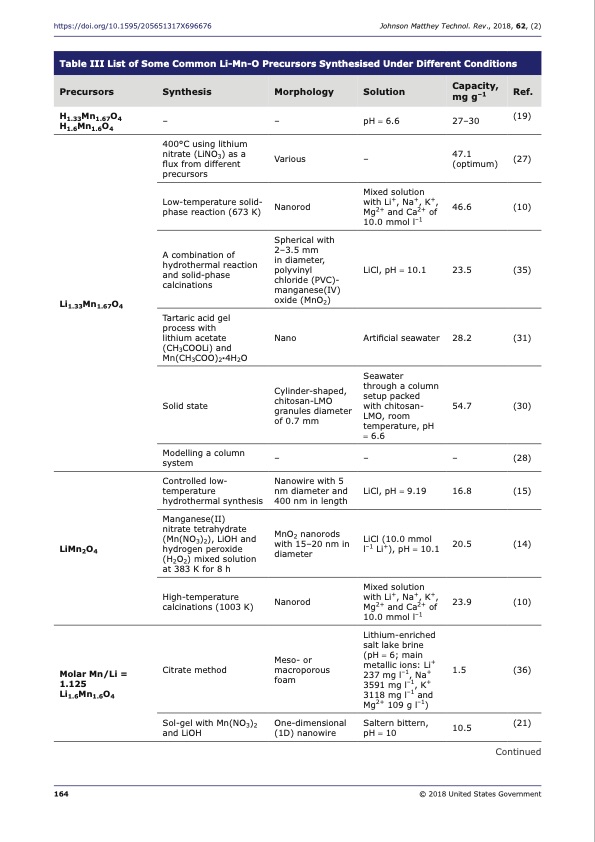
https://doi.org/10.1595/205651317X696676 Johnson Matthey Technol. Rev., 2018, 62, (2) Table III List of Some Common Li-Mn-O Precursors Synthesised Under Different Conditions Precursors Synthesis Morphology Solution Capacity, Ref. mg g–1 H1.33Mn1.67O4 H1.6Mn1.6O4 – 400°C using lithium nitrate (LiNO3) as a flux from different precursors Low-temperature solid- phase reaction (673 K) A combination of hydrothermal reaction and solid-phase calcinations Tartaric acid gel process with lithium acetate (CH3COOLi) and Mn(CH3COO)2·4H2O Solid state Modelling a column system Controlled low- temperature hydrothermal synthesis Manganese(II) nitrate tetrahydrate (Mn(NO3)2), LiOH and hydrogen peroxide (H2O2) mixed solution at 383 K for 8 h High-temperature calcinations (1003 K) Citrate method Sol-gel with Mn(NO3)2 and LiOH – Various Nanorod Spherical with 2–3.5 mm in diameter, polyvinyl chloride (PVC) manganese(IV) oxide (MnO2) Nano Cylinder-shaped, chitosan-LMO granules diameter of 0.7 mm – Nanowire with 5 nm diameter and 400 nm in length MnO2 nanorods with 15–20 nm in diameter Nanorod Meso- or macroporous foam One-dimensional (1D) nanowire pH = 6.6 27–30 (19) – 47.1 (27) (optimum) Li1.33Mn1.67O4 Mixed solution with Li+, Na+, K+, 46.6 Mg2+ and Ca2+ of 10.0 mmol l–1 LiCl, pH = 10.1 23.5 Artificial seawater 28.2 Seawater through a column setup packed with chitosan- 54.7 LMO, room temperature, pH = 6.6 – – LiCl, pH = 9.19 16.8 LiCl (10.0 mmol 20.5 l–1 Li+), pH = 10.1 Mixed solution with Li+, Na+, K+, 23.9 Mg2+ and Ca2+ of 10.0 mmol l–1 Lithium-enriched salt lake brine (pH = 6; main + metallic ions: Li 1.5 237 mg l–1, Na+ 3591 mg l–1, K+ 3118 mg l–1 and Mg2+ 109 g l–1) Saltern bittern, 10.5 pH = 10 (10) (35) (31) (30) (28) (15) (14) (10) (36) (21) Continued LiMn2O4 Molar Mn/Li = 1.125 Li1.6Mn1.6O4 164 © 2018 United States GovernmentPDF Image | Lithium Recovery from Aqueous Resources
PDF Search Title:
Lithium Recovery from Aqueous ResourcesOriginal File Name Searched:
b8befda967a8ccf19190203d3b5aeae0673f.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |