
PDF Publication Title:
Text from PDF Page: 007
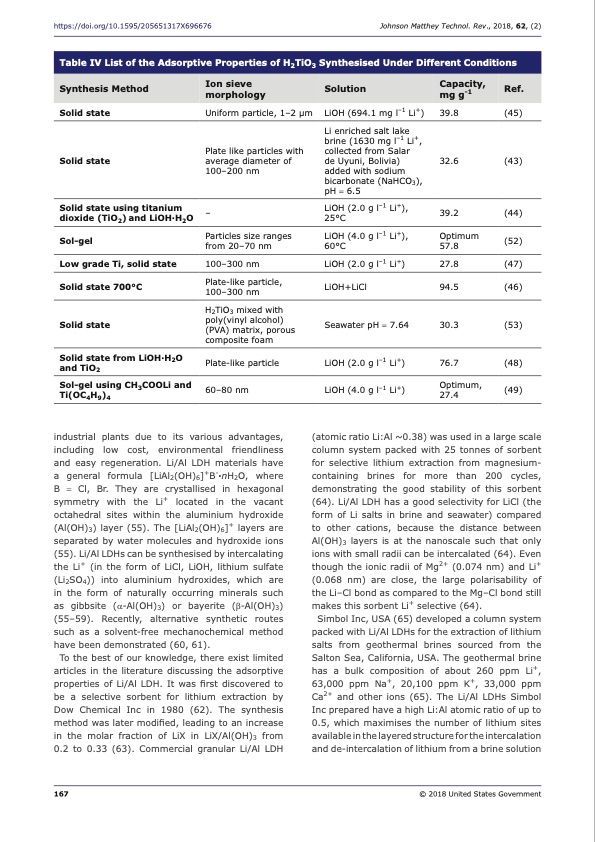
https://doi.org/10.1595/205651317X696676 Johnson Matthey Technol. Rev., 2018, 62, (2) Table IV List of the Adsorptive Properties of H2TiO3 Synthesised Under Different Conditions Synthesis Method Ion sieve Solution Capacity, morphology mg g-1 Ref. Solid state Solid state Solid state using titanium dioxide (TiO2) and LiOH·H2O Sol-gel Low grade Ti, solid state Solid state 700°C Solid state Solid state from LiOH·H2O and TiO2 Sol-gel using CH3COOLi and Ti(OC4H9)4 Uniform particle, 1–2 μm Plate like particles with average diameter of 100–200 nm – Particles size ranges from 20–70 nm 100–300 nm Plate-like particle, 100–300 nm H2TiO3 mixed with poly(vinyl alcohol) (PVA) matrix, porous composite foam Plate-like particle 60–80 nm LiOH (694.1 mg l–1 Li+) Li enriched salt lake brine (1630 mg l–1 Li+, collected from Salar de Uyuni, Bolivia) added with sodium bicarbonate (NaHCO3), pH = 6.5 LiOH (2.0 g l–1 Li+), 25°C LiOH (2.0 g l–1 Li+) LiOH+LiCl Seawater pH = 7.64 LiOH (2.0 g l–1 Li+) LiOH (4.0 g l–1 Li+) 39.8 (45) 32.6 (43) 39.2 (44) Optimum (52) 60°C 57.8 LiOH (4.0 g l–1 Li+), 27.8 (47) 94.5 (46) 30.3 (53) 76.7 (48) Optimum, (49) 27.4 industrial plants due to its various advantages, including low cost, environmental friendliness and easy regeneration. Li/Al LDH materials have a general formula [LiAl2(OH)6]+B-·nH2O, where B = Cl, Br. They are crystallised in hexagonal symmetry with the Li+ located in the vacant octahedral sites within the aluminium hydroxide (Al(OH)3) layer (55). The [LiAl2(OH)6]+ layers are separated by water molecules and hydroxide ions (55). Li/Al LDHs can be synthesised by intercalating the Li+ (in the form of LiCl, LiOH, lithium sulfate (Li2SO4)) into aluminium hydroxides, which are in the form of naturally occurring minerals such as gibbsite (α-Al(OH)3) or bayerite (β-Al(OH)3) (55–59). Recently, alternative synthetic routes such as a solvent-free mechanochemical method have been demonstrated (60, 61). To the best of our knowledge, there exist limited articles in the literature discussing the adsorptive properties of Li/Al LDH. It was first discovered to be a selective sorbent for lithium extraction by Dow Chemical Inc in 1980 (62). The synthesis method was later modified, leading to an increase in the molar fraction of LiX in LiX/Al(OH)3 from 0.2 to 0.33 (63). Commercial granular Li/Al LDH (atomic ratio Li:Al ~0.38) was used in a large scale column system packed with 25 tonnes of sorbent for selective lithium extraction from magnesium- containing brines for more than 200 cycles, demonstrating the good stability of this sorbent (64). Li/Al LDH has a good selectivity for LiCl (the form of Li salts in brine and seawater) compared to other cations, because the distance between Al(OH)3 layers is at the nanoscale such that only ions with small radii can be intercalated (64). Even though the ionic radii of Mg2+ (0.074 nm) and Li+ (0.068 nm) are close, the large polarisability of the Li–Cl bond as compared to the Mg–Cl bond still makes this sorbent Li+ selective (64). Simbol Inc, USA (65) developed a column system packed with Li/Al LDHs for the extraction of lithium salts from geothermal brines sourced from the Salton Sea, California, USA. The geothermal brine has a bulk composition of about 260 ppm Li+, 63,000 ppm Na+, 20,100 ppm K+, 33,000 ppm Ca2+ and other ions (65). The Li/Al LDHs Simbol Inc prepared have a high Li:Al atomic ratio of up to 0.5, which maximises the number of lithium sites available in the layered structure for the intercalation and de-intercalation of lithium from a brine solution 167 © 2018 United States GovernmentPDF Image | Lithium Recovery from Aqueous Resources
PDF Search Title:
Lithium Recovery from Aqueous ResourcesOriginal File Name Searched:
b8befda967a8ccf19190203d3b5aeae0673f.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |