
PDF Publication Title:
Text from PDF Page: 004
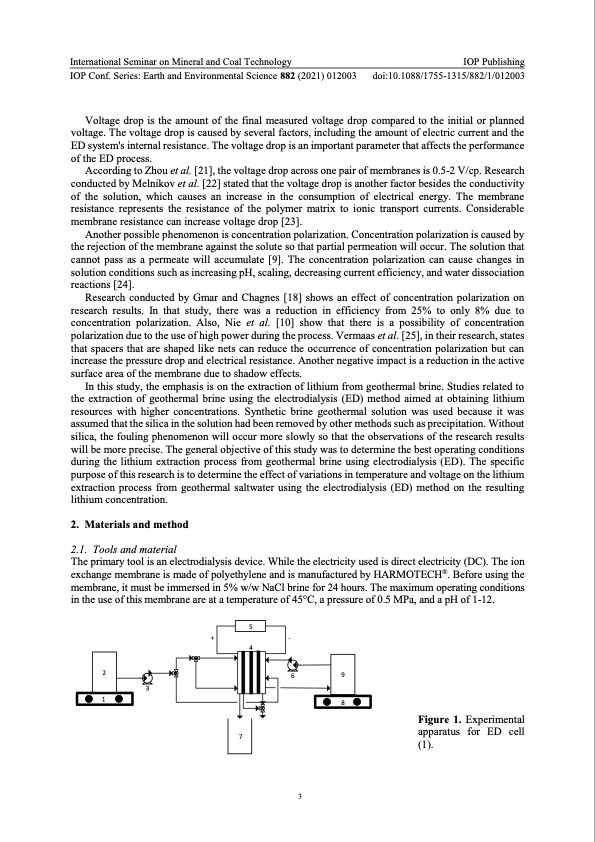
International Seminar on Mineral and Coal Technology IOP Publishing IOP Conf. Series: Earth and Environmental Science 882 (2021) 012003 doi:10.1088/1755-1315/882/1/012003 Voltage drop is the amount of the final measured voltage drop compared to the initial or planned voltage. The voltage drop is caused by several factors, including the amount of electric current and the ED system's internal resistance. The voltage drop is an important parameter that affects the performance of the ED process. According to Zhou et al. [21], the voltage drop across one pair of membranes is 0.5-2 V/cp. Research conducted by Melnikov et al. [22] stated that the voltage drop is another factor besides the conductivity of the solution, which causes an increase in the consumption of electrical energy. The membrane resistance represents the resistance of the polymer matrix to ionic transport currents. Considerable membrane resistance can increase voltage drop [23]. Another possible phenomenon is concentration polarization. Concentration polarization is caused by the rejection of the membrane against the solute so that partial permeation will occur. The solution that cannot pass as a permeate will accumulate [9]. The concentration polarization can cause changes in solution conditions such as increasing pH, scaling, decreasing current efficiency, and water dissociation reactions [24]. Research conducted by Gmar and Chagnes [18] shows an effect of concentration polarization on research results. In that study, there was a reduction in efficiency from 25% to only 8% due to concentration polarization. Also, Nie et al. [10] show that there is a possibility of concentration polarization due to the use of high power during the process. Vermaas et al. [25], in their research, states that spacers that are shaped like nets can reduce the occurrence of concentration polarization but can increase the pressure drop and electrical resistance. Another negative impact is a reduction in the active surface area of the membrane due to shadow effects. In this study, the emphasis is on the extraction of lithium from geothermal brine. Studies related to the extraction of geothermal brine using the electrodialysis (ED) method aimed at obtaining lithium resources with higher concentrations. Synthetic brine geothermal solution was used because it was assumed that the silica in the solution had been removed by other methods such as precipitation. Without silica, the fouling phenomenon will occur more slowly so that the observations of the research results will be more precise. The general objective of this study was to determine the best operating conditions during the lithium extraction process from geothermal brine using electrodialysis (ED). The specific purpose of this research is to determine the effect of variations in temperature and voltage on the lithium extraction process from geothermal saltwater using the electrodialysis (ED) method on the resulting lithium concentration. 2. Materials and method 2.1. Tools and material The primary tool is an electrodialysis device. While the electricity used is direct electricity (DC). The ion exchange membrane is made of polyethylene and is manufactured by HARMOTECH®. Before using the membrane, it must be immersed in 5% w/w NaCl brine for 24 hours. The maximum operating conditions in the use of this membrane are at a temperature of 45°C, a pressure of 0.5 MPa, and a pH of 1-12. 2 1 3 5 +- 4 7 69 8 3 Figure 1. Experimental apparatus for ED cell (1).PDF Image | Lithium recovery synthetic geothermal brine electrodialysis
PDF Search Title:
Lithium recovery synthetic geothermal brine electrodialysisOriginal File Name Searched:
lithium-from-brine-geothermal.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |