
PDF Publication Title:
Text from PDF Page: 004
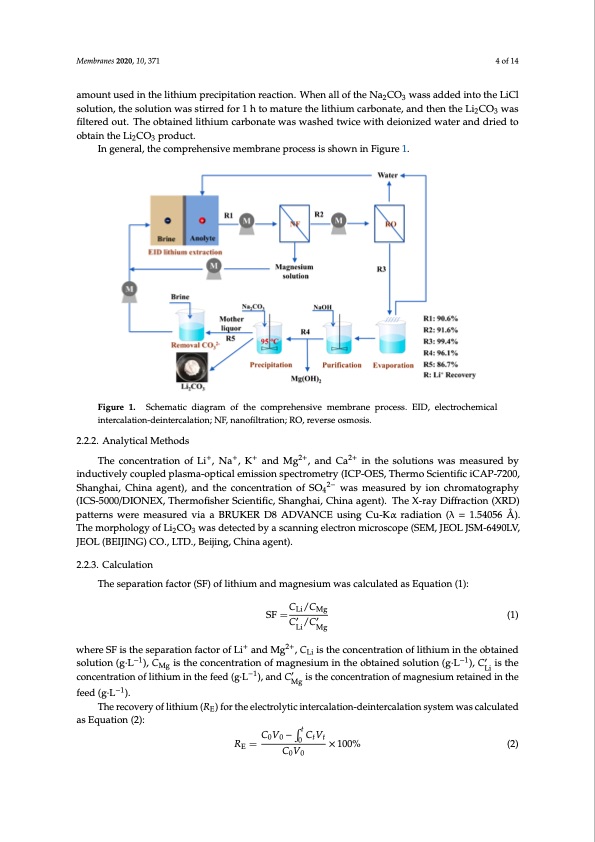
Membranes 2020, 10, 371 4 of 14 amount used in the lithium precipitation reaction. When all of the Na2CO3 wass added into the LiCl Membranes 2020, 10, x FOR PEER REVIEW 4 of 14 solution, the solution was stirred for 1 h to mature the lithium carbonate, and then the Li2CO3 was filtered out. The obtained lithium carbonate was washed twice with deionized water and dried to Li2CO3 was filtered out. The obtained lithium carbonate was washed twice with deionized water and obtain the Li CO product. dried to obta2in th3e Li2CO3 product. In general, the comprehensive membrane process is shown in Figure 1. In general, the comprehensive membrane process is shown in Figure 1. Figure 1. Schematic diagram of the comprehensive membrane process. EID, electrochemical Figure 1. Schematic diagram of the comprehensive membrane process. EID, electrochemical intercalation-deintercalation; NF, nanofiltration; RO, reverse osmosis. intercalation-deintercalation; NF, nanofiltration; RO, reverse osmosis. 2.2.2. Analytical Methods 2.2.2. Analytical Methods The concentration of Li+, Na+, K+ and Mg2+, and Ca2+ in the solutions was measured by The concentration of Li+, Na+, K+ and Mg2+, and Ca2+ in the solutions was measured by inductively inductively coupled plasma-optical emission spectrometry (ICP-OES, Thermo Scientific iCAP-7200, coupled plasma-optical emission spectrometry (ICP-OES, Thermo Scientific iCAP-7200, Shanghai, Shanghai, China agent), and the concentration of SO42− was measured by ion chromatography China agent), and the concentration of SO42− was measured by ion chromatography (ICS- (ICS-5000/DIONEX, Thermofisher Scientific, Shanghai, China agent). The X-ray Diffraction (XRD) 5000/DIONEX, Thermofisher Scientific, Shanghai, China agent). The X-Ray Diffraction (XRD) patterns were measured via a BRUKER D8 ADVANCE using Cu-Kα radiation (λ = 1.54056 Å). patterns were measured via a BRUKER D8 ADVANCE using Cu-Kα radiation (λ = 1.54056 Å). The The morphology of Li2CO3 was detected by a scanning electron microscope (SEM, JEOL JSM-6490LV, morphology of Li2CO3 was detected by a scanning electron microscope (SEM, JEOL JSM-6490LV, JEOL (BEIJING) CO., LTD., Beijing, China agent). JEOL (BEIJING) CO., LTD., Beijing, China agent). 2.2.3. Calculation 2.2.3. Calculation The separation factor (SF) of lithium and magnesium was calculated as Equation (1): The separation factor (SF) of lithium and magnesium was calculated as Equation (1): C /C Li/ Mg SF=Li Mg (1) SF=C′ /C′ (1) calculated as Equation (2): as Equation (2): Mg −1 ′ −1 ’ ′ /′ Li Mg CV−CV 00 tt Li Mg + 2+ whereSFistthesseeparrattiionffaaccttorrooffLLii+andMg2+,,CLi iissttheconcentrationoflithiumintheobtained −1 −1 ′ solution (g··L−1)),, CMg isistthee concenttrattiion of magnesium in the obtained sollutiion ((g··L−1),, CLi’ is the Li concenttrattiionooffliltihthiuiummininthtehefefeeded(g·(Lg·L),)a,nadndCCMgisitshtehceoncocnencetrnatriaotnionf mofamgnaegsniuemsiurmetarienteadiniendthine Mg thefeed(g·L ). feed (g·L−1). −1 The recovery of lithium (RE) for the electrolytic intercalation-deintercalation system was The recovery of lithium (RE) for the electrolytic intercalation-deintercalation system was calculated t Li − 0 R = ×100% (2) E CV (2) = ×100% 00 where RE is the recovery of lithium in the brine, C0 is the initial concentration of lithium in the brine (g·L−1), V0 is the initial volume of the brine (L), t is the sampling time (h), Ct is the concentration of lithium in brine at t (g·L−1), and Vt is the volume of brine at t (L).PDF Image | Membrane Process for Preparing Lithium Carbonate
PDF Search Title:
Membrane Process for Preparing Lithium CarbonateOriginal File Name Searched:
membranes-10-00371.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |