
PDF Publication Title:
Text from PDF Page: 006
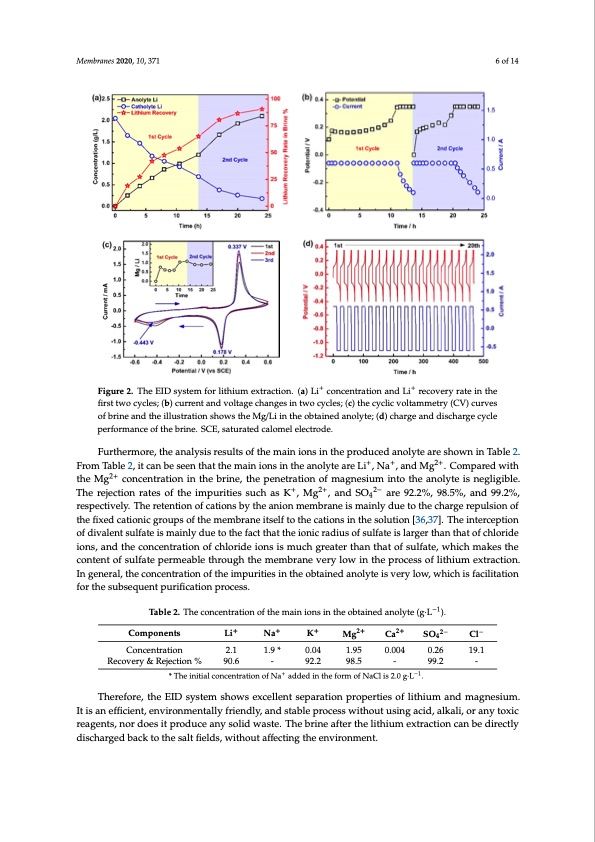
Membranes 2020, 10, 371 6 of 14 Membranes 2020, 10, x FOR PEER REVIEW 6 of 14 Figure2.TheEIDsystemforlithiumextraction.(a)L+i+concentrationand+Li+recoveryrateinthe Figure 2. The EID system for lithium extraction. (a) Li concentration and Li recovery rate in the first first two cycles; (b) current and voltage changes in two cycles; (c) the cyclic voltammetry (CV) curves two cycles; (b) current and voltage changes in two cycles; (c) the cyclic voltammetry (CV) curves of of brine and the illustration shows the Mg/Li in the obtained anolyte; (d) charge and discharge cycle brine and the illustration shows the Mg/Li in the obtained anolyte; (d) charge and discharge cycle performance of the brine. SCE, saturated calomel electrode. performance of the brine. SCE, saturated calomel electrode. Furthermore, the analysis results of the main ions in the produced anolyte are shown in Table 2. Furthermore, the analysis results of the main ions in the produced anolyte are shown in Table 2. From Table 2, it can be seen that the main ions in the anolyte are Li+, Na+, and Mg2+. Compared with From Table 2, it can be seen that the main ions in the anolyte are Li+, Na+, and Mg2+. Compared with the Mg2+ concentration in the brine, the penetration of magnesium into the anolyte is negligible. the Mg2+ concentration in the brine, the penetration of magnesium into the anolyte is negligible. The The rejection rates of the impurities such as K+, Mg2+, and SO 2− are 92.2%, 98.5%, and 99.2%, + 2+ 2− 4 rejectionratesoftheimpuritiessuchasK,Mg ,andSO4 are92.2%,98.5%,and99.2%,respectively. respectively. The retention of cations by the anion membrane is mainly due to the charge repulsion of The retention of cations by the anion membrane is mainly due to the charge repulsion of the fixed the fixed cationic groups of the membrane itself to the cations in the solution [36,37]. The interception cationic groups of the membrane itself to the cations in the solution [36,37]. The interception of of divalent sulfate is mainly due to the fact that the ionic radius of sulfate is larger than that of chloride divalent sulfate is mainly due to the fact that the ionic radius of sulfate is larger than that of chloride ions, and the concentration of chloride ions is much greater than that of sulfate, which makes the ions, and the concentration of chloride ions is much greater than that of sulfate, which makes the content of sulfate permeable through the membrane very low in the process of lithium extraction. content of sulfate permeable through the membrane very low in the process of lithium extraction. In In general, the concentration of the impurities in the obtained anolyte is very low, which is facilitation general, the concentration of the impurities in the obtained anolyte is very low, which is facilitation for the subsequent purification process. for the subsequent purification process. Table 2. The concentration of the main ions in the obtained anolyte (g·L−1). Table 2. The concentration of the main ions in the obtained anolyte (g·L−1). Components Na+ SO 2− Cl− SO42− 4 Cl− 0.26 19.1 Li+ 2.1 Na+ Li+ K+ Mg2+ K+ Mg2+ Ca2+ Ca2+ - * The initial concentration of Na+ added in the form of NaCl is 2.0 g·L−1. Components Concentration 1.9 * 0.04 1.95 0.004 Recovery & Rejection % 90.6 - 92.2 98.5 99.2 - Concentration 2.1 1.9 * - 0.04 1.95 0.004 0.26 19.1 Recovery & Rejection % * The initial concentration of Na+ added in the form of NaCl is 2.0 g·L−1. 90.6 92.2 98.5 - 99.2 - Therefore, the EID system shows excellent separation properties of lithium and magnesium. Therefore, the EID system shows excellent separation properties of lithium and magnesium. It It is an efficient, environmentally friendly, and stable process without using acid, alkali, or any toxic is an efficient, environmentally friendly, and stable process without using acid, alkali, or any toxic reagents, nor does it produce any solid waste. The brine after the lithium extraction can be directly reagents, nor does it produce any solid waste. The brine after the lithium extraction can be directly discharged back to the salt fields, without affecting the environment. discharged back to the salt fields, without affecting the environment.PDF Image | Membrane Process for Preparing Lithium Carbonate
PDF Search Title:
Membrane Process for Preparing Lithium CarbonateOriginal File Name Searched:
membranes-10-00371.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |