
PDF Publication Title:
Text from PDF Page: 011
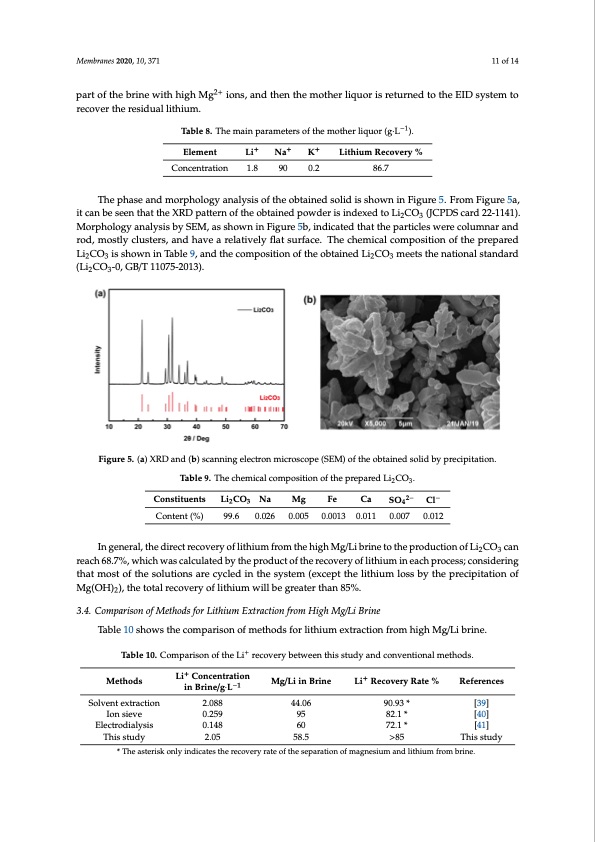
Element Concentration Li+ Na+ K+ 1.8 90 0.2 Lithium Recovery % 86.7 Table 8. The main parameters of the mother liquor (g·L−1). Membranes 2020, 10, 371 The phase and morphology analysis of the obtained solid is shown in Figure 5. From Figure 5a, it can be seen that the XRD pattern of the obtained powder is indexed to Li2CO3 (JCPDS card 22-1141). 2+ MpaorrtpohfotlhoegbyrainealwysiitshbhyigShEM,gas sihoonws,naindFtihgeunreth5eb,minodthiceartelidqtuhoartitshreeptuarntiecdlestowtehreEcoIDlumsynstaermantdo roecdo,vmerotshtleyrcelsuidstuearls,liathnidumha.ve a relatively flat surface. The chemical composition of the prepared Li2CO3 is shown in Table 9, and the composition of the obtained Li2CO3 meets the national standard Table 8. The main parameters of the mother liquor (g·L−1). (Li2CO3-0, GB/T 11075-2013). Element Li+ Na+ K+ Lithium Recovery % Table 9. The chemical composition of the prepared Li2CO3. 11 of 14 Concentration 1.8 Constituents Li2CO3 Na 90 0.2 Mg 0.005 Fe 0.0013 86.7 Ca SO42− Cl− 0.012 Content (%) 99.6 0.026 0.011 0.007 The phase and morphology analysis of the obtained solid is shown in Figure 5. From Figure 5a, it can be seen that the XRD pattern of the obtained powder is indexed to Li2CO3 (JCPDS card 22-1141). In general, the direct recovery of lithium from the high Mg/Li brine to the production of Li2CO3 Morphology analysis by SEM, as shown in Figure 5b, indicated that the particles were columnar and can reach 68.7%, which was calculated by the product of the recovery of lithium in each process; rod, mostly clusters, and have a relatively flat surface. The chemical composition of the prepared considering that most of the solutions are cycled in the system (except the lithium loss by the Li2CO3 is shown in Table 9, and the composition of the obtained Li2CO3 meets the national standard precipitation of Mg(OH)2), the total recovery of lithium will be greater than 85%. (Li2CO3-0, GB/T 11075-2013). Figure 5. (a) XRD and (b) scanning electron microscope (SEM) of the obtained solid by precipitation. Figure 5. (a) XRD and (b) scanning electron microscope (SEM) of the obtained solid by precipitation. Table 9. The chemical composition of the prepared Li2CO3. 3.4. Comparison of Methods for Lithium Extraction from High Mg/Li Brine Constituents Li2CO3 Na Mg Fe Ca SO42− Cl− Table 10 shows the comparison of methods for lithium extraction from high Mg/Li brine. Content (%) 99.6 0.026 0.005 0.0013 0.011 0.007 0.012 Table 10. Comparison of the Li+ recovery between this study and conventional methods. In general, the direct recovery of lithium from the high Mg/Li brine to the production of Li2CO3 can Li+ Concentration in Mg/Li in Li+ Recovery reachM68e.7t%ho,dwshichwascalculatedbytheproductoftherecoveryoflithiumineachprocessR;ceofenrseidnecreisng Brine/g·L−1 Brine Rate % that most of the solutions are cycled in the system (except the lithium loss by the precipitation of Solvent Mg(OH)2), the total recovery of l2it.h0i8u8m will be greater t4h4a.n0685%. Table 10 shows the comparison of methods for lithium extraction from high Mg/Li brine. This study 2.05 58.5 >85 This study extraction Ion sieve 0.259 95 90.93 * [39] 82.1 * [40] 72.1 * [41] 3.4. Comparison of Methods for Lithium Extraction from High Mg/Li Brine Electrodialysis 0.148 60 * The asterisk only indicates the recovery rate of the separation of magnesium and lithium from brine. Table 10. Comparison of the Li+ recovery between this study and conventional methods. ++ From Table 10, it Lcai nCboensceenntratthioant the total Li recovery rate in this paper is superior to that of Methods in Brine/g·L−1 Mg/Li in Brine Li+ Recovery Rate % References ion sieve method and electrolysis method, but slightly lower than that of solvent extraction method. HoSwoleveenrt,etxhteraectxitornactant used2.0in88the solvent extr4a4c.t0i6on method has a 9s0li.g93ht* dissolution in[t3h9e] brine, Ion sieve Electrodialysis This study 0.259 95 0.148 60 82.1 * 72.1 * >85 [40] [41] This study 2.05 58.5 * The asterisk only indicates the recovery rate of the separation of magnesium and lithium from brine.PDF Image | Membrane Process for Preparing Lithium Carbonate
PDF Search Title:
Membrane Process for Preparing Lithium CarbonateOriginal File Name Searched:
membranes-10-00371.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |