
PDF Publication Title:
Text from PDF Page: 006
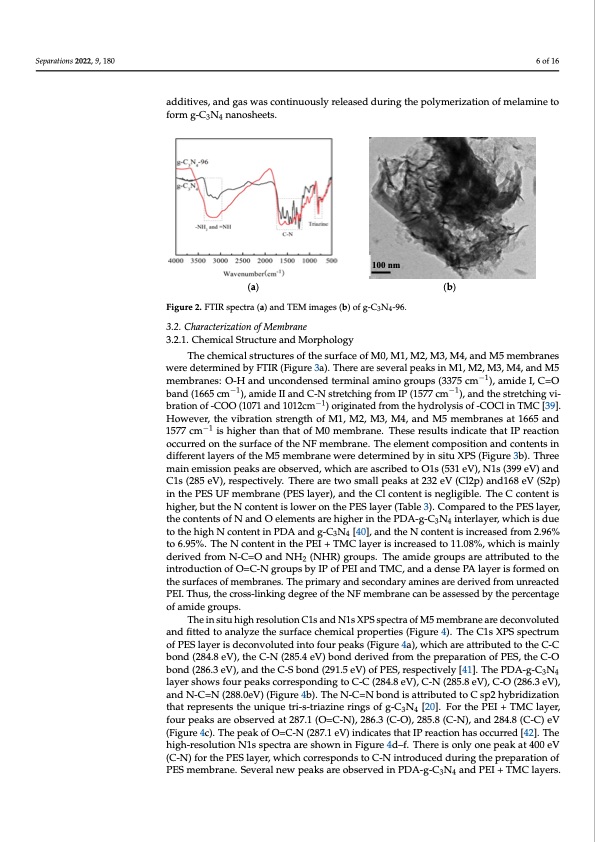
Separations 2022, 9, 180 The FTIR spectra of g-C3N4-96 are shown in Figure 2a. In the spectrum of g-C3N4, the absorption peak at 802 cm−1 corresponds to the characteristic breathing mode of triazine units, while those peaks in the range of 1234–1637 cm−1 are assigned to the stretching vi- bration of C-N and C=N heterocycles, which are similar to those reported in previous studies [36,37]. The broad peak in the range of 3000–3500 cm−1 is owed to the stretching 6 of 16 vibrations of N-H or N-H2 originated from uncondensed amino groups [38]. In Figure 2b, a typical ultrathin nanosheets-like architecture with a crinkly structure is observed for g- C3N4-96. This is because urea-inorganic ammonium salts were used as additives, and gas additives, and gas was continuously released during the polymerization of melamine to was continuously released during the polymerization of melamine to form g-C3N4 form g-C3N4 nanosheets. nanosheets. (a) (b) Figure 2. FTIR spectra (a) and TEM images (b) of g-C3N4-96. Figure 2. FTIR spectra (a) and TEM images (b) of g-C3N4-96. 3.2. Characterization of Membrane 3.2. Characterization of Membrane 3.2.1. Chemical Structure and Morphology 3.2.1. Chemical Structure and Morphology 100 nm The chemical structures of the surface of M0, M1, M2, M3, M4, and M5 membranes The chemical structures of the surface of M0, M1, M2, M3, M4, and M5 membranes were determined by FTIR (Figure 3a). There are several peaks in M1, M2, M3, M4, and M5 were determined by FTIR (Figure 3a). There are several peaks in M1, M2, M3, M4, and M5 membranes: O-H and uncondensed terminal amino groups (3375 cm−1), amide I, C=O membranes: O-H and uncondensed terminal amino groups (3375 cm−1), amide I, C=O band (1665 cm−1), amide II and C-N stretching from IP (1577 cm−1), and the stretching vi- band (1665 cm−1), amide II and C-N stretching from IP (1577 cm−1), and the stretching vi- bration of -COO (1071 and 1012cm−1) originated from the hydrolysis of -COCl in TMC [39]. bration of -COO (1071 and 1012cm−1) originated from the hydrolysis of -COCl in TMC [39]. However, the vibration strength of M1, M2, M3, M4, and M5 membranes at 1665 and However, the vibration strength of M1, M2, M3, M4, and M5 membranes at 1665 and 1577 1577 cm−1 is higher than that of M0 membrane. These results indicate that IP reaction cm−1 is higher than that of M0 membrane. These results indicate that IP reaction occurred occurred on the surface of the NF membrane. The element composition and contents in on the surface of the NF membrane. The element composition and contents in different different layers of the M5 membrane were determined by in situ XPS (Figure 3b). Three main emission peaks are observed, which are ascribed to O1s (531 eV), N1s (399 eV) and C1s (285 eV), respectively. There are two small peaks at 232 eV (Cl2p) and168 eV (S2p) in the PES UF membrane (PES layer), and the Cl content is negligible. The C content is higher, but the N content is lower on the PES layer (Table 3). Compared to the PES layer, the contents of N and O elements are higher in the PDA-g-C3N4 interlayer, which is due to the high N content in PDA and g-C3N4 [40], and the N content is increased from 2.96% to 6.95%. The N content in the PEI + TMC layer is increased to 11.08%, which is mainly derived from N-C=O and NH2 (NHR) groups. The amide groups are attributed to the introduction of O=C-N groups by IP of PEI and TMC, and a dense PA layer is formed on the surfaces of membranes. The primary and secondary amines are derived from unreacted PEI. Thus, the cross-linking degree of the NF membrane can be assessed by the percentage of amide groups. The in situ high resolution C1s and N1s XPS spectra of M5 membrane are deconvoluted and fitted to analyze the surface chemical properties (Figure 4). The C1s XPS spectrum of PES layer is deconvoluted into four peaks (Figure 4a), which are attributed to the C-C bond (284.8 eV), the C-N (285.4 eV) bond derived from the preparation of PES, the C-O bond (286.3 eV), and the C-S bond (291.5 eV) of PES, respectively [41]. The PDA-g-C3N4 layer shows four peaks corresponding to C-C (284.8 eV), C-N (285.8 eV), C-O (286.3 eV), and N-C=N (288.0eV) (Figure 4b). The N-C=N bond is attributed to C sp2 hybridization that represents the unique tri-s-triazine rings of g-C3N4 [20]. For the PEI + TMC layer, four peaks are observed at 287.1 (O=C-N), 286.3 (C-O), 285.8 (C-N), and 284.8 (C-C) eV (Figure 4c). The peak of O=C-N (287.1 eV) indicates that IP reaction has occurred [42]. The high-resolution N1s spectra are shown in Figure 4d–f. There is only one peak at 400 eV (C-N) for the PES layer, which corresponds to C-N introduced during the preparation of PES membrane. Several new peaks are observed in PDA-g-C3N4 and PEI + TMC layers.PDF Image | Nanofiltration Membrane Using Polydopamine Carbon Nitride
PDF Search Title:
Nanofiltration Membrane Using Polydopamine Carbon NitrideOriginal File Name Searched:
separations-09-00180.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |