
PDF Publication Title:
Text from PDF Page: 009
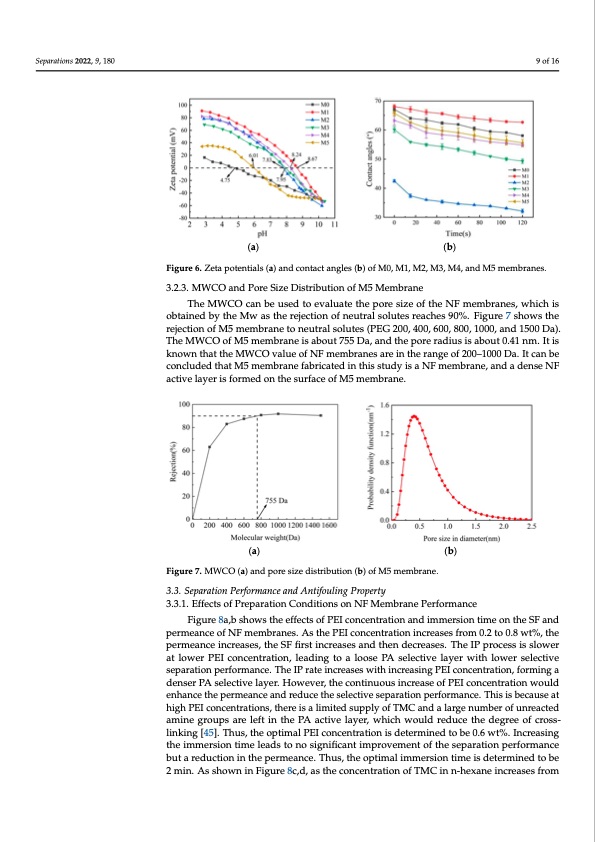
Separations 2022, 9, 180 > M2. It should be noted that the WCAs of the M1 membrane are always higher than that of other membranes, indicating that the PA NF membrane is less hydrophilic. The g-C3N4 in the PEI aqueous phase forms hydrogen bonds with water molecules, which enhances the affinity of water molecules with the membrane surface and reduces the WCAs of M2 membrane [22]. The hydrophilicity can be improved by adding g-C3N4 or depositing DA 9 of 16 on the surface of the base membrane. The WCAs of the M5 membrane decrease because of the formation of the PDA-g-C3N4 interlayer. (a) (b) Figure 6. Zeta potentials (a) and contact angles (b) of M0, M1, M2, M3, M4, and M5 membranes. Figure 6. Zeta potentials (a) and contact angles (b) of M0, M1, M2, M3, M4, and M5 membranes. 3.2.3. MWCO and Pore Size Distribution of M5 Membrane 3.2.3. MWCO and Pore Size Distribution of M5 Membrane The MWCO can be used to evaluate the pore size of the NF membranes, which is The MWCO can be used to evaluate the pore size of the NF membranes, which is obtained by the Mw as the rejection of neutral solutes reaches 90%. Figure 7 shows the obtained by the Mw as the rejection of neutral solutes reaches 90%. Figure 7 shows the rejection of M5 membrane to neutral solutes (PEG 200, 400, 600, 800, 1000, and 1500 Da). rejection of M5 membrane to neutral solutes (PEG 200, 400, 600, 800, 1000, and 1500 Da). The MWCO of M5 membrane is about 755 Da, and the pore radius is about 0.41 nm. It is The MWCO of M5 membrane is about 755 Da, and the pore radius is about 0.41 nm. It is known that the MWCO value of NF membranes are in the range of 200–1000 Da. It can be known that the MWCO value of NF membranes are in the range of 200–1000 Da. It can be Separations2022,9,xFORPEERREVIEWconcludedthatM5membranefabricatedinthisstudyisaNFmembrane,andad10enosfe17NF concluded that M5 membrane fabricated in this study is a NF membrane, and a dense NF active layer is formed on the surface of M5 membrane. active layer is formed on the surface of M5 membrane. (a) (b) Figure 7. MWCO (a) and pore size distribution (b) of M5 membrane. Figure 7. MWCO (a) and pore size distribution (b) of M5 membrane. 3.3. Separation Performance and Antifouling Property 3.3. Separation Performance and Antifouling Property 3.3.1. Effects of Preparation Conditions on NF Membrane Performance 3.3.1. Effects of Preparation Conditions on NF Membrane Performance Figure 8a,b shows the effects of PEI concentration and immersion time on the SF and Figure 8a,b shows the effects of PEI concentration and immersion time on the SF and permeance of NF membranes. As the PEI concentration increases from 0.2 to 0.8 wt%, the permeance of NF membranes. As the PEI concentration increases from 0.2 to 0.8 wt%, the permeance increases, the SF first increases and then decreases. The IP process is slower permeance increases, the SF first increases and then decreases. The IP process is slower at at lower PEI concentration, leading to a loose PA selective layer with lower selective lower PEI concentration, leading to a loose PA selective layer with lower selective separa- separation performance. The IP rate increases with increasing PEI concentration, forming a tion performance. The IP rate increases with increasing PEI concentration, forming a denser PA selective layer. However, the continuous increase of PEI concentration would denser PA selective layer. However, the continuous increase of PEI concentration would enhance the permeance and reduce the selective separation performance. This is because at enhance the permeance and reduce the selective separation performance. This is because high PEI concentrations, there is a limited supply of TMC and a large number of unreacted at high PEI concentrations, there is a limited supply of TMC and a large number of unre- amine groups are left in the PA active layer, which would reduce the degree of cross- acted amine groups are left in the PA active layer, which would reduce the degree of cross- linking [45]. Thus, the optimal PEI concentration is determined to be 0.6 wt%. Increasing linking [45]. Thus, the optimal PEI concentration is determined to be 0.6 wt%. Increasing the immersion time leads to no significant improvement of the separation performance the immersion time leads to no significant improvement of the separation performance but a reduction in the permeance. Thus, the optimal immersion time is determined to be but a reduction in the permeance. Thus, the optimal immersion time is determined to be 2 min. As shown in Figure 8c,d, as the concentration of TMC in n-hexane increases from 2 min. As shown in Figure 8c,d, as the concentration of TMC in n-hexane increases from 0.05 to 0.4 wt%, the separation performance first increases and then decreases, while the permeance gradually decreases. This is because there is no sufficient TMC to react with PEI at low TMC concentrations and the IP is weak. However, once the optimum IP is achieved, the increasing of the TMC concentration would actually decrease the separation performance, because the hydrolysis of unreacted acid chloride groups in TMC to car-PDF Image | Nanofiltration Membrane Using Polydopamine Carbon Nitride
PDF Search Title:
Nanofiltration Membrane Using Polydopamine Carbon NitrideOriginal File Name Searched:
separations-09-00180.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |