
PDF Publication Title:
Text from PDF Page: 011
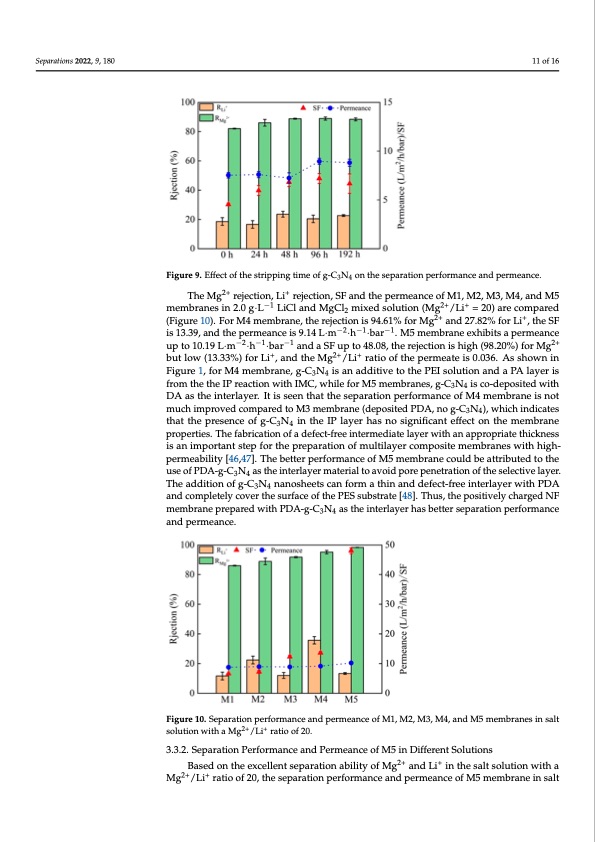
Separations 2022, 9, 180 Figure 8. Effects of fabrication conditions on membrane separation performance and permeance. (a) 11 of 16 PEI concentration; (b) immersion time of PEI; (c) TMC concentration; (d) reaction time; (e) g-C3N4 concentration; (f) immersion time of DA. Figure 9. Effect of the stripping time of g-C3N4 on the separation performance and permeance. Figure 9. Effect of the stripping time of g-C3N4 on the separation performance and permeance. The Mg2+ rejection, Li+ rejection, SF and the permeance of M1, M2, M3, M4, and M5 Separations 2022, 9, x FOR PEER REVIEW 2+ + 12 of 17 The Mg rejectio−n1, Li rejection, SF and the permeance o2f+M1,+M2, M3, M4, and M5 membranes in 2.0 g·L LiCl and MgCl2 mixed solution (Mg /Li = 20) are compared −1 2+ + membranes in 2.0 g·L LiCl and MgCl2 mixed solution (Mg 2/+Li = 20) are compa+red (Fig- (Figure 10). For M4 membrane, the rejection is 94.61% for Mg and 27.82% for Li , the SF ure 10). For M4 membrane, the rejecti−on2 is−914.61−%1 for Mg2+ and 27.82% for Li+, the SF is is 13.39, and the permeance is 9.14 L·m ·h ·bar . M5 membrane exhibits a permeance −2−1 −1 −2−1 −1 2+ 103..139,La·mnd t·he·pb−ear2rme−aan1ndceai−SsF19.u14pLto·m48·.h08,·bthaer r.eMjec5timonemisbhraignhe e(9x8h.i2b0i%ts)afpoerrMmgeanbcuetulpow2t+o up to 10.19 L·m ·h ·bar and a SF up to 48.08, the rejection is high (98.20%) for Mg (13.33%) for Li+, and the+Mg2+/Li+ ratio2+of th+e permeate is 0.036. As shown in Figure 1, for but low (13.33%) for Li , and the Mg /Li ratio of the permeate is 0.036. As shown in M4 membrane, g-C3N4 is an additive to the PEI solution and a PA layer is from the the IP Figure 1, for M4 membrane, g-C3N4 is an additive to the PEI solution and a PA layer is reaction with IMC, while for M5 membranes, g-C3N4 is co-deposited with DA as the in- from the the IP reaction with IMC, while for M5 membranes, g-C3N4 is co-deposited with terlayer. It is seen that the separation performance of M4 membrane is not much improved DA as the interlayer. It is seen that the separation performance of M4 membrane is not compared to M3 membrane (deposited PDA, no g-C3N4), which indicates that the pres- much improved compared to M3 membrane (deposited PDA, no g-C3N4), which indicates ence of g-C3N4 in the IP layer has no significant effect on the membrane properties. The that the presence of g-C3N4 in the IP layer has no significant effect on the membrane fabrication of a defect-free intermediate layer with an appropriate thickness is an im- properties. The fabrication of a defect-free intermediate layer with an appropriate thickness ipsoarntanimtpstoerptafnotrsttheeppfroerptahreatpiorenpoaframtiuolntiloafymeruclotimlapyoersicteompeomsbitreanmeesmwbirthanheisghw-iptherhmigeah- pbielritmye[a4b6i,l4i7ty].[T4h6,e47b]e.tTtehrepbeertftoerrmpaenrcfoeromfaMnc5emofemMb5rmanemcborualndebceouatltdribeutaetdtritbouttheedutosethoef uPsDeAo-fgP-DC3AN-g4-aCstNheianstethrleaiynetrermlaayterimalattoeraivalotiodapvooriedppeonretrpaetnioentraotfiothneosfetlheectsievleclatiyverl.aTyheer. 34 Tadhdeiatidodnitoiofng-oCf3gN-4CnaNnosnhaeneotshceaentsfocarmnfaortmhinaathnidndanefdecdt-efrecet-ifnreterilnayterlawyietrhwPiDthAPaDnAd 34 aconmd pcolemtepllyetceolyvecrovtheer tshuersfaucrefaocef tohfethPeEPSEsSusbusbtrsatrteat[e4[84]8. ]T. hTuhsu,st,htheeppoossitiitviveelylycharged NF membranepreparedwithPDA-g-C3N4 astheiinterlayerhasbetterseparationperformance and permeance. 34 Figure 10. Separation performance and permeance of M1, M2, M3, M4, and M5 membranes in salt Figure 10. Separation performance and permeance of M1, M2, M3, M4, and M5 membranes in salt 2+ + solution with a Mg2+ /L+i ratio of 20. solutionwithaMg /Li ratioof20. 3.3.2. Separation Performance and Permeance of M5 in Different Solutions 3.3.2. Separation Performance and Permeance of M5 in Different Solutions Based on the excellent separation ability of Mg2+ and Li+ in the salt solution with a 2+Base+d on the excellent separation ability of Mg2+ and Li+ in the salt solution with a Mg /Li ratio of 20, the separation performance and permeance of M5 membrane in salt Mg2+/Li+ ratio of 20, the separation performance and permeance of M5 membrane in salt solutions with a Mg2+/Li+ ratio of 48 and 81 are examined. It is found that at a Mg2+/Li+ ratio of 48, the SF of M5 membrane is 15.14 and the permeance is 8.60 L·m−2·h−1·bar−1; while at a Mg2+/Li+ ratio of 84, the SF is 10.77 and the permeance is 7.51 L·m−2·h−1·bar−1 (Figure 11). The SF of the M5 membrane decreases with the increase of the Mg2+/Li+ ratio, but the re-PDF Image | Nanofiltration Membrane Using Polydopamine Carbon Nitride
PDF Search Title:
Nanofiltration Membrane Using Polydopamine Carbon NitrideOriginal File Name Searched:
separations-09-00180.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |