
PDF Publication Title:
Text from PDF Page: 004
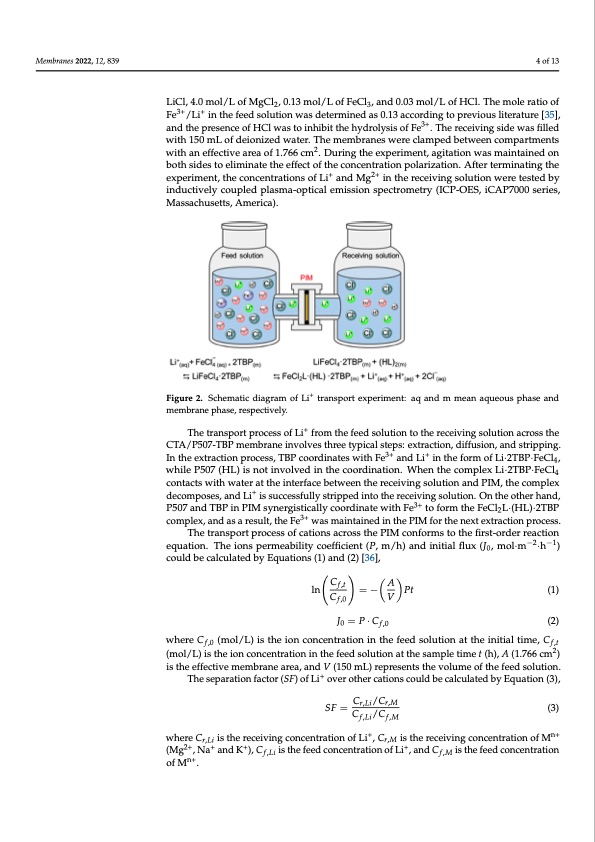
Fe3+/Li+ in the feed solution was determined as 0.13 according to previous literature [35], and the presence of HCl was to inhibit the hydrolysis of Fe3+. The receiving side was filled with 150 mL of deionized water. The membranes were clamped between compartments with an effective area of 1.766 cm2. During the experiment, agitation was maintained on both sides to eliminate the effect of the concentration polarization. After terminating the Membranes 2022, 12, 839 + 2+ experiment, the concentrations of Li and Mg in the receiving solution were tested by inductively coupled plasma-optical emission spectrometry (ICP-OES, iCAP7000 series, Massachusetts, America). 4 of 13 The tranLsipCol,rt4.p0rmocoels/sLooffLMi+gfrColm,0th.1e3fmeeodl/sLolouftiFoenCtlo,tahnedr0e.c0e3ivminogl/sLolouftiHonCla.cTrhosesmoleratioof 23 the CTA/P50F7-eTB/PLmi eimn bthraenfeeindvsollvuetsiotnhrweeastydpeitcearlmstienpesd: eaxst0ra.1c3tiaocnc,odridffiungsioton,parnedvisoturispl-iterature [35], 3+ 3+ + ping. In theanedxttrhaectpiorensepnrcoecoefssH,CTlBwPascotorindhinibaittetshewhityhdrFoelysisanodfFLei .iTnhethrecefoivrimngosfidewasfilled 3+ + Li·2TBP·FeClw4,itwhh1i5le0Pm5L07of(HdLei)oinsizneodtwinavtoelrv.eTdheinmthemebcoraonrdesinwateiorenc.lWamhpenedthbetcwomeepnlecxompartments 2 Li·2TBP·FeClw4citohnatanctesffwecithivweatreraaotfth1e.7i6n6tecrmfac.eDbuetrwinegenthtehexrpeceerivminengts,oalguitiaotnioannwdaPsIMm,aintainedon + the complex bdoetchomsidpeosetos,ealnimdiLniaties tshuecceeffsescftuollfythstericpopnecdenintrtaotitohne preocleairvizinagtiosnol.uAtifotenr. Otenrminating the next extraction process. experiment, the concentrations of Li+ and Mg2+ in the receiving solution were tested by the other hand, P507 and TBP in PIM synergistically coordinate with Fe3+ to form the inductively coupled plasma-optical em3+ission spectrometry (ICP-OES, iCAP7000 series, FeCl2L·(HL)·2TBP complex, and as a result, the Fe was maintained in the PIM for the Massachusetts, America). + Figure 2. ScheFmigatuicred2ia.gSracmheomfaLtictdraiangspraomrt eoxfpLeirimtreannts:paqoratnedxpmermimeaenta:qauqeoaunsdpmhasmeeaanndamqeumeo-us phase and + branephase,rmesepmecbtrivaenleyp.hase,respectively. where 𝐶 , (mol/L) is the ion concentration in the feed solution at the initial time, 𝐶 The transport process of cations across the PIM conforms to the first-o,rder reaction (3), The separation factor (SF) of Li+ over other cations could be calculated by Equation Cf,t A The transport process of Li+ from the feed solution to the receiving solution across the The transport process of cations across the PIM conforms to the first-order reaction CTA/P507-TBP membrane involves three typical steps: extraction−,2 dif−f1usion, and stripping. equation. The ions permeability coefficient (P, m/h) and initial flux (J0, mol·m · h ) could In the extraction process, TBP coordinates with Fe3+ and Li+ in the form of Li·2TBP·FeCl , be calculated by Equation (1) and Equation (2) [36], 4 while P507 (HL) is not involved in the coordination. When the complex Li·2TBP·FeCl4 𝐶, 𝐴 contacts with water at the interface between the receiving solution and PIM, the complex + 𝑙𝑛 𝐶 𝑉 𝑃𝑡 (1) decomposes, and Li is succes,sfully stripped into the receiving solution. On the other hand, P507 and TBP in PIM synergistically coordinate with Fe3+ to form the FeCl L·(HL)·2TBP 𝐽 𝑃⋅𝐶 (2) 3+ , 2 complex, and as a result, the Fe was maintained in the PIM for the next extraction process. 2 −2−1 (mol/L)istheqiounatcionc.eTnthreationsinpethrmefeeaebdilsitoyluctoioenffiactiethnet(sPa,mmp/leht)imanedt(ihn)i,tiAal(fl1.u7x66(Jcm,m)ol·m ·h ) 0 is the effectivceomuledmbbercaanlecualraetae,danbyd EVq(u15a0tiomnLs)(r1e)parnedse(n2t)s[3th6e],volume of the feed solution. =− V Pt (1) J0 =P·Cf,0 (2) where Cf,0 (mol/L) is the ion concentration in the feed solution at the initial time, Cf,t (mol/L) is the ion concentration in the feed solution at the sample time t (h), A (1.766 cm2) is the effective membrane area, and V (150 mL) represents the volume of the feed solution. The separation factor (SF) of Li+ over other cations could be calculated by Equation (3), SF = Cr,Li/Cr,M (3) Cf,Li/Cf,M where Cr,Li is the receiving concentration of Li+, Cr,M is the receiving concentration of Mn+ (Mg2+,Na+ andK+),Cf,Li isthefeedconcentrationofLi+,andCf,M isthefeedconcentration of Mn+. ln C f,0PDF Image | P507 TBP Carriers for Lithium Extraction from Brines
PDF Search Title:
P507 TBP Carriers for Lithium Extraction from BrinesOriginal File Name Searched:
membranes-12-00839.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |