
PDF Publication Title:
Text from PDF Page: 005
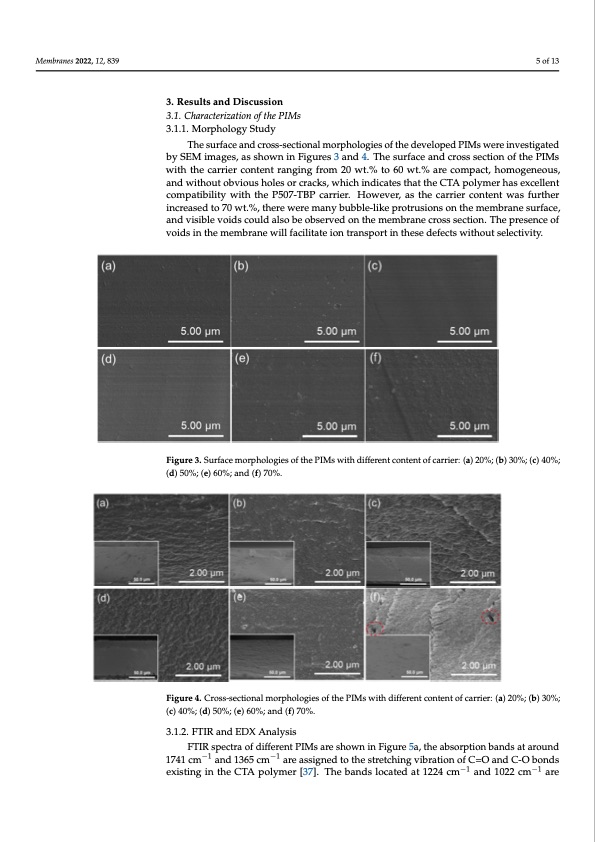
𝑆𝐹 𝐶 /𝐶 (3) (3) where 𝐶 where 𝐶, 𝐶,/𝐶, , , is the receiving concentration of Li+, 𝐶 is the receiving concentration of is the receiving concentration of Li , 𝐶, is the receiving concentration of , + , M n+(Mg 2+, Na +and K +), 𝐶 is the feed concentration of Li+ , and 𝐶 is the feed concen- M (Mg , Na and K ), 𝐶, is the feed concentration of Li , and 𝐶, is the feed concen- n+ 2+ + + , + , tration of Mn+. tration of Mn+. Membranes 2022, 12, 839 33. .ReessullttssaandDiiscussion 33.1.1. .Chhaarraacctteerriizzattiion off the PIMs 3. Results and Discussion 5 of 13 3 3 . 1. 1 . 1. 1 . . M M o o r r p p h h o o l l o o g g y y S S t t u u d d y y 3.1. Characterization of the PIMs T T h h e e s s u u r r f f a a c c e e a a n n d d c c r r o o s s s s - - s s e e c c t t i i o o n n a a l l m m o o r r p p h h o o l l o o g g i i e e s s o o f f t t h h e e d d e e v v e e l l o o p p e e d d P P I I M M s s w w e e r r e e i ni n v v e e s s t i t - i - 3.1.1. Morphology Study g g a a t t e e d d b b y y S S E E M M i i m m a a g g e e s s , , a a s s s s h h o o w w n n i i n n F F i i g g u u r r e e s s 3 3 a a n n d d 4 4 . . T T h h e e s s u u r r f f a a c c e e a a n n d d c c r r o o s s s s s s e e c c t t i oi o n n o o f f t ht h e e The surface and cross-sectional morphologies of the developed PIMs were investigated PPIIMsswiitthtthecarrriiercontentranging from 20 wt.%to60wt..%arreccompaacctt,,hhoomooggeennee-- by SEM images, as shown in Figures 3 and 4. The surface and cross section of the PIMs oouuss,,aanndwiitthouwttoiotbhbvtihioeuscsahrroilelerscornctcernactckrsa,nwgihnigchfrioinmdi2c0atwest.t%thattotth6he0eCwCTtT.A%Apaporoellyycmomeerprhahacasts,ehexox-m-ogeneous, cceellelennttccoompattiibaiilnliidttywwitihithohuthoebPv5io0u7s-ThBoPlescaorrcierar.ckHso,owhevivcehr,i,nadsitcthahetecscatrhrariietertrhcceoCntTteAenntptwowlayasmsfeufurrr-h-as excellent ther increased toco7m0 wpatt.%ibi,ltihtyerweiwthertehemPa5n0y7b-TuBbPblcea-lrirkier.prHotorwuseivoenrs, oans the mcaerrmiebrraconnetseunrt-was further ther increased to 70 wt.%, there were many bubble-like protrusions on the membrane sur- face, and visibleinvcoreidasecdotuold70awlsot.%b,ethoebrserwverde omnanthyebmubebmleb-lriakneepcrorotrsusssioenctsion. tThhe empermesb-rane surface, face, and visible voids could also be observed on the membrane cross section. The pres- and visible voids could also be observed on the membrane cross section. The presence of ence of voids in the membrane will facilitate ion transport in these defects without selec- ence of voids in the membrane will facilitate ion transport in these defects without selec- tivity. tivity. voids in the membrane will facilitate ion transport in these defects without selectivity. Figure 3. Surface morphologies of the PIMs with different content of carrier: (a) 20%; (b) 30%; (c) Figure 3. Surface morphologies of the PIMs with different content of carrier: (a) 20%; (b) 30%; (c) 40%; Figure 3. Surface morphologies of the PIMs with different content of carrier: (a) 20%; (b) 30%; (c) 40%; (d) 50%; (e) 60%; and (f) 70%. Figure 4. Cross-sectional morphologies of the PIMs with different content of carrier: (a) 20%; (b) (d) 50%; (e) 60%; and (f) 70%. 40%; (d) 50%; (e) 60%; and (f) 70%. Figure 4. Cross-sectional morphologies of the PIMs with different content of carrier: (a) 20%; (b) 30%; (c) 40%; (d) 50%; (e) 60%; and (f) 70%. Figure 4. Cross-sectional morphologies of the PIMs with different content of carrier: (a) 20%; (b) 30%; 30%; (c) 40%; (d) 50%; (e) 60%; and (f) 70%. (c) 40%; (d) 50%; (e) 60%; and (f) 70%. 3.1.2. FTIR and EDX Analysis FTIR spectra of different PIMs are shown in Figure 5a, the absorption bands at around 1741 cm−1 and 1365 cm−1 are assigned to the stretching vibration of C=O and C-O bonds existing in the CTA polymer [37]. The bands located at 1224 cm−1 and 1022 cm−1 arePDF Image | P507 TBP Carriers for Lithium Extraction from Brines
PDF Search Title:
P507 TBP Carriers for Lithium Extraction from BrinesOriginal File Name Searched:
membranes-12-00839.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |