
PDF Publication Title:
Text from PDF Page: 008
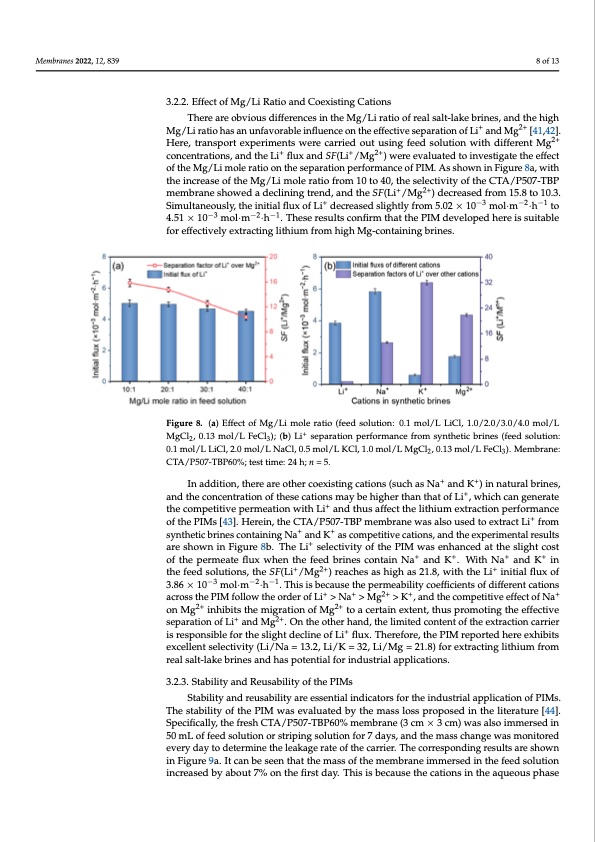
Membranes 2022, 12, 839 10.3. Simultaneously, the initial flux of Li+ decreased slightly from 5.02 × 10−3 mol·m−2·h−1 to 4.51 × 10−3 mol·m−2·h−1. These results confirm that the PIM developed here is suitable for effectively extracting lithium from high Mg-containing brines. In addition, there are other coexisting cations (such as Na+ and K+) in natural brines, and the concentration of these cations may be higher than that of Li+, which can generate the competitive permeation with Li+ and thus affect the lithium extraction performance of the PIMs [43]. Herein, the CTA/P507-TBP membrane was also used to extract Li+ from 3.2.2. Effect of Mg/Li Ratio and Coexisting Cations sults are shown in Figure 8b. The Li+ selectivity of the PIM was enhanced at the slight cost There are obvious differences in the Mg/Li ratio of real salt-lake brines, and the high ++++ ofthepermeatefluxwhenthefeedbrinescontainNa andK.WithN+a andK2+inthefeed Mg/Li ratio has an unfavorable influence on the effective separation of Li and Mg [41,42]. solutions, the SF(Li+/Mg2+) reaches as high as 21.8, with the Li+ initial flux of 3.86 ×2+10−3 8 of 13 synthetic brines containing Na+ and K+ as competitive cations, and the experimental re- Here, transport experiments were carried out using feed solution with different Mg mol·m−2·h−1. This is becau+se the permeab+ility 2c+oefficients of different cations across the concentrations, and the Li flux and SF(Li /Mg ) were evaluated to investigate the effect PIM follow the order of Li+ > Na+ > Mg2+ > K+, and the competitive effect of Na+ on Mg2+ of the Mg/Li mole ratio on the separation performance of PIM. As shown in Figure 8a, with 2+ inthiebintscrtehaesemoigfrthateioMngo/fLMimgolteoratcioerftraoimne1x0tetont4,0t,htuhsepserloemctoivtitnygotfhtehefCfeTcAti/veP5s0e7p-aTrBaPtion + 2+ +2+ ofmLeimabnrdanMesgho.wOendtahedeoctlhinerinhgatnrden,dth,eanlidmtihtedSFco(Lnite/nMt ogf th)edecxrterascetdiofnrocmarr1i5e.r8itsor1e0sp.3o.n- + −3 −2−1 siSbilme ufoltrantheeousslilgy,htthde eincliitniael flouf xLioffLlui x.dTechreraesfeodres,litghetlPyIfMromrep5.o0r2te×d1h0eremexohl·imbits·ehxcetlolent 4.51 × 10−3 mol·m−2·h−1. These results confirm that the PIM developed here is suitable selectivity (Li/Na = 13.2, Li/K = 32, Li/Mg = 21.8) for extracting lithium from real salt-lake + for effectively extracting lithium from high Mg-containing brines. brines and has potential for industrial applications. Figure 8. (a) Effect of Mg/Li mole ratio (feed solution: 0.1 mol/L LiCl, 1.0/2.0/3.0/4.0 mol/L MgCl2, Figure 8. (a) Effect of Mg/Li mole ratio (feed solution: 0.1 mol/L LiCl, 1.0/2.0/3.0/4.0 mol/L 0.13 mol/L FeCl3); (b) Li+ separation+ performance from synthetic brines (feed solution: 0.1 mol/L MgCl2, 0.13 mol/L FeCl3); (b) Li separation performance from synthetic brines (feed solution: LiCl, 2.0 mol/L NaCl, 0.5 mol/L KCl, 1.0 mol/L MgCl2, 0.13 mol/L FeCl3). Membrane: CTA/P507- 0.1 mol/L LiCl, 2.0 mol/L NaCl, 0.5 mol/L KCl, 1.0 mol/L MgCl2, 0.13 mol/L FeCl3). Membrane: TBP60%; test time: 24 h; n = 5. CTA/P507-TBP60%; test time: 24 h; n = 5. ++ In addition, there are other coexisting cations (such as Na and K ) in natural brines, 3.2.3. Stability and reusability of the PIMs and the concentration of these cations may be higher than that of Li+, which can generate Stability and reusability are essential indicators for the industrial application of PIMs. the competitive permeation with Li+ and thus affect the lithium extraction performance The stability of the PIM was evaluated by the mass loss proposed in the literature [44]. of the PIMs [43]. Herein, the CTA/P507-TBP membrane was also used to extract Li+ from Specifically, the fresh CTA/P507-TBP60% membrane (3 cm × 3 cm) was also immersed in synthetic brines containing Na+ and K+ as competitive cations, and the experimental results 50 mL of feed solution or striping solution for 7 days, and the mass change was monitored are shown in Figure 8b. The Li+ selectivity of the PIM was enhanced at the slight cost every day to determine the leakage rate of the carrier. The corresponding results are of the permeate flux when the feed brines contain Na+ and K+. With Na+ and K+ in shown in Figure 9a. It can be seen that the mass of the membrane immersed in the feed the feed solutions, the SF(Li+/Mg2+) reaches as high as 21.8, with the Li+ initial flux of solution in−c3reased b−y2 ab−o1ut 7% on the first day. This is because the cations in the aqueous 3.86 × 10 mol·m ·h . This is because the permeability coefficients of different cations phase were extracted into the memb+rane p+hase, a2+nd th+e extraction process reached a p+lat- across the PIM follow the order of Li > Na > Mg > K , and the competitive effect of Na eau with2+in 24 h owing to the limitatio2n+ of carrier content. No obvious mass difference of on Mg inhibits the migration of Mg to a certain extent, thus promoting the effective separation of Li+ and Mg2+. On the other hand, the limited content of the extraction carrier is responsible for the slight decline of Li+ flux. Therefore, the PIM reported here exhibits excellent selectivity (Li/Na = 13.2, Li/K = 32, Li/Mg = 21.8) for extracting lithium from real salt-lake brines and has potential for industrial applications. 3.2.3. Stability and Reusability of the PIMs Stability and reusability are essential indicators for the industrial application of PIMs. The stability of the PIM was evaluated by the mass loss proposed in the literature [44]. Specifically, the fresh CTA/P507-TBP60% membrane (3 cm × 3 cm) was also immersed in 50 mL of feed solution or striping solution for 7 days, and the mass change was monitored every day to determine the leakage rate of the carrier. The corresponding results are shown in Figure 9a. It can be seen that the mass of the membrane immersed in the feed solution increased by about 7% on the first day. This is because the cations in the aqueous phasePDF Image | P507 TBP Carriers for Lithium Extraction from Brines
PDF Search Title:
P507 TBP Carriers for Lithium Extraction from BrinesOriginal File Name Searched:
membranes-12-00839.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |