
PDF Publication Title:
Text from PDF Page: 009
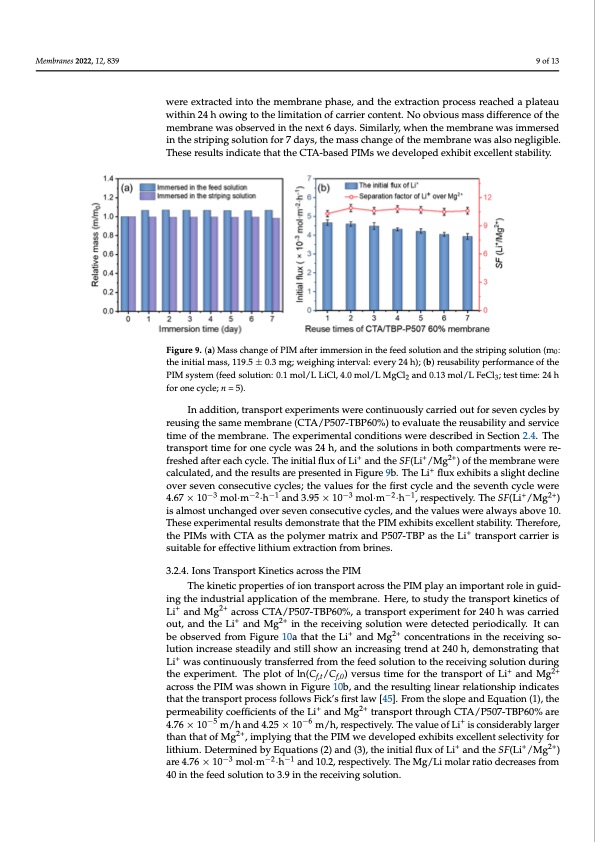
Membranes 2022, 12, 839 In addition, transport experiments were continuously carried out for seven cycles by reusing the same membrane (CTA/P507-TBP60%) to evaluate the reusability and service time of the membrane. The experimental conditions were described in Section 2.4. The transport time for one cycle was 24 h, and the solutions in both compartments were re- freshed after each cycle. The initial flux of Li+ and the SF(Li+/Mg2+) of the membran9 eofw13ere calculated, and the results are presented in Figure 9b. The Li+ flux exhibits a slight decline over seven consecutive cycles; the values for the first cycle and the seventh cycle were 4.67 −3 −2−1 −3 −2−1 +2+ ×w10eremexotlr·macte·dhinatnodth3e.9m5e×m1b0ranmeopl·hmase·h,an,dretshpeecetxitvrealcyti.oTnhperSoFce(Lssir/Meagche)disaaplmlaotesatuun- chwainthgiend2o4vherowseivnegntocothneselicmuittiavteiocnycolfecsa,rarniedr cthoentveanltu. eNsowoebrveioaulws mayasssabdoifvfere1n0c.eTohfetsheeex- membrane was observed in the next 6 days. Similarly, when the membrane was immersed perimental results demonstrate that the PIM exhibits excellent stability. Therefore, the in the striping solution for 7 days, the mass change of the mem+brane was also negligible. PIMswithCTAasthepolymermatrixandP507-TBPastheLi transportcarrierissuitable These results indicate that the CTA-based PIMs we developed exhibit excellent stability. for effective lithium extraction from brines. Figure 9. (a) Mass change of PIM after immersion in the feed solution and the striping solution (m0: Figure 9. (a) Mass change of PIM after immersion in the feed solution and the striping solution (m0: the initial mass, 119.5 ± 0.3 mg; weighing interval: every 24 h); (b) reusability performance of the the initial mass, 119.5 ± 0.3 mg; weighing interval: every 24 h); (b) reusability performance of the PIM system (feed solution: 0.1 mol/L LiCl, 4.0 mol/L MgCl2 and 0.13 mol/L FeCl3; test time: 24 h for PIM system (feed solution: 0.1 mol/L LiCl, 4.0 mol/L MgCl2 and 0.13 mol/L FeCl3; test time: 24 h one cycle; n = 5). for one cycle; n = 5). 3.2.4. IonnasdTdriatinosnp, otratnKspinoertiecxspaecriomsesnthtsewPeIrMe continuously carried out for seven cycles by reusing the same membrane (CTA/P507-TBP60%) to evaluate the reusability and service The kinetic properties of ion transport across the PIM play an important role in guid- time of the membrane. The experimental conditions were described in Section 2.4. The ing the industrial application of the membrane. Here, to study the transport kinetics of Li+ transport time for one cycle was 24 h, and the solutions in both compartments were re- and Mg2+ across CTA/P507-TBP60%, a transport experiment for 240 h was carried out, and freshed after each cycle. The initial flux of Li+ and the SF(Li+/Mg2+) of the membrane were the Li+ and Mg2+ in the receiving solution were detected periodically. It can be observed calculated, and the results are presented in Figure 9b. The Li+ flux exhibits a slight decline from Figure 10a that the Li+ and Mg2+ concentrations in the receiving solution increase over seven consecutive cycles; the values for the first cycle and the seventh cycle were steadily an−d3 still sh−o2w a−n1 increasing tre−n3d at 240−2h, d−e1monstrating that Li+ wa+s con2t+inu- 4.67 × 10 mol·m ·h and 3.95 × 10 mol·m ·h , respectively. The SF(Li /Mg ) ously transferred from the feed solution to the receiving solution during the experiment. is almost unchanged over seven consecutive cycles, and the values were always above 10. The plot of ln(Cf,t/Cf,0) versus time for the transport of Li+ and Mg2+ across the PIM was These experimental results demonstrate that the PIM exhibits excellent stability. Therefore, + shown in Figure 10b, and the resulting linear relationship indicates that the transport pro- the PIMs with CTA as the polymer matrix and P507-TBP as the Li transport carrier is cessusitfaoblloewfosrFeifcfekc’tsivfierslitthlaiuwm[4e5x]t.rFacrtoiomntfhroemslobprieneasn.d Equation (1), the permeability coeffi- cients of the Li+ and Mg2+ transport through CTA/P507-TBP60% are 4.76 × 10−5 m/h and 3.2.4. Ions Transport Kinetics across the PIM 4.25 × 10−6 m/h, respectively. The value of Li+ is considerably larger than that of Mg2+, im- The kinetic properties of ion transport across the PIM play an important role in guid- plying that the PIM we developed exhibits excellent selectivity for lithium. Determined ingtheindustrialapplicationofthemembrane.H+ere,tostudythe+tran2s+portkineticsof −3 by Equations (2) and (3), the initial flux of Li and the SF(Li /Mg ) are 4.76 × 10 Li+an−2dM−1g2+acrossCTA/P507-TBP60%,atransportexperimentfor240hwascarried mol·m ·h and 10.2, respectively. The Mg/Li molar ratio decreases from 40 in the feed out, and the Li+ and Mg2+ in the receiving solution were detected periodically. It can solution to 3.9 in the receiving solution. + 2+ be observed from Figure 10a that the Li and Mg In addition, the separation performance comparison of different liquid membranes lution increase steadily and still show an increasing trend at 240 h, demonstrating that is shown in Table 2. The CTA/P507-TBP60% membrane exhibits higher Li+ selectivity than Li+ was continuously transferred from the feed solution to the receiving solution during most other membranes, and the permeability is comparable to other PIM membranes the experiment. The plot of ln(Cf,t/Cf,0) versus time for the transport of Li+ and Mg2+ across the PIM was shown in Figure 10b, and the resulting linear relationship indicates that the transport process follows Fick’s first law [45]. From the slope and Equation (1), the permeability coefficients of the Li+ and Mg2+ transport through CTA/P507-TBP60% are 4.76 × 10−5 m/h and 4.25 × 10−6 m/h, respectively. The value of Li+ is considerably larger than that of Mg2+, implying that the PIM we developed exhibits excellent selectivity for lithium. Determined by Equations (2) and (3), the initial flux of Li+ and the SF(Li+/Mg2+) are 4.76 × 10−3 mol·m−2·h−1 and 10.2, respectively. The Mg/Li molar ratio decreases from 40 in the feed solution to 3.9 in the receiving solution. concentrations in the receiving so-PDF Image | P507 TBP Carriers for Lithium Extraction from Brines
PDF Search Title:
P507 TBP Carriers for Lithium Extraction from BrinesOriginal File Name Searched:
membranes-12-00839.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |