
PDF Publication Title:
Text from PDF Page: 009
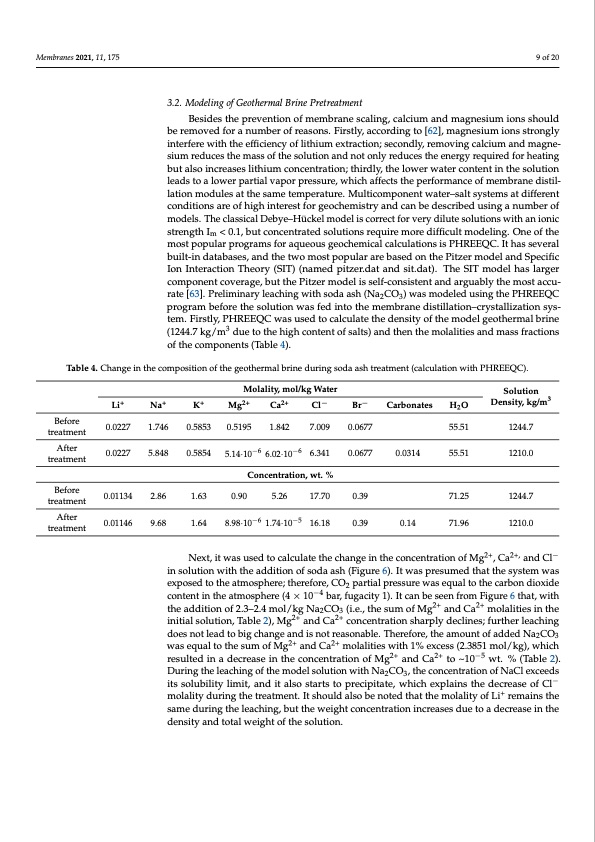
Membranes 2021, 11, 175 9 of 20 3.2. Modeling of Geothermal Brine Pretreatment Besides the prevention of membrane scaling, calcium and magnesium ions should be removed for a number of reasons. Firstly, according to [62], magnesium ions strongly interfere with the efficiency of lithium extraction; secondly, removing calcium and magne- sium reduces the mass of the solution and not only reduces the energy required for heating but also increases lithium concentration; thirdly, the lower water content in the solution leads to a lower partial vapor pressure, which affects the performance of membrane distil- lation modules at the same temperature. Multicomponent water–salt systems at different conditions are of high interest for geochemistry and can be described using a number of models. The classical Debye–Hückel model is correct for very dilute solutions with an ionic strength Im < 0.1, but concentrated solutions require more difficult modeling. One of the most popular programs for aqueous geochemical calculations is PHREEQC. It has several built-in databases, and the two most popular are based on the Pitzer model and Specific Ion Interaction Theory (SIT) (named pitzer.dat and sit.dat). The SIT model has larger component coverage, but the Pitzer model is self-consistent and arguably the most accu- rate [63]. Preliminary leaching with soda ash (Na2CO3) was modeled using the PHREEQC program before the solution was fed into the membrane distillation–crystallization sys- tem. Firstly, PHREEQC was used to calculate the density of the model geothermal brine (1244.7 kg/m3 due to the high content of salts) and then the molalities and mass fractions of the components (Table 4). Table 4. Change in the composition of the geothermal brine during soda ash treatment (calculation with PHREEQC). Molality, mol/kg Water Li+ Na+ K+ Mg2+ Ca2+ Cl− Br− Carbonates Solution H2O Density, kg/m3 55.51 1244.7 55.51 1210.0 71.25 1244.7 71.96 1210.0 Before treatment After treatment Before treatment After treatment 0.0227 1.746 0.0227 5.848 0.01134 2.86 0.01146 9.68 0.5853 0.5195 1.842 7.009 0.0677 6.341 0.0677 0.5854 5.14·10−6 6.02·10−6 Concentration, wt. % 0.0314 0.14 1.63 0.90 5.26 17.70 0.39 1.64 8.98·10−6 1.74·10−5 16.18 0.39 Next, it was used to calculate the change in the concentration of Mg2+, Ca2+, and Cl− in solution with the addition of soda ash (Figure 6). It was presumed that the system was exposed to the atmosphere; therefore, CO2 partial pressure was equal to the carbon dioxide content in the atmosphere (4 × 10−4 bar, fugacity 1). It can be seen from Figure 6 that, with the addition of 2.3–2.4 mol/kg Na2CO3 (i.e., the sum of Mg2+ and Ca2+ molalities in the initial solution, Table 2), Mg2+ and Ca2+ concentration sharply declines; further leaching does not lead to big change and is not reasonable. Therefore, the amount of added Na2CO3 was equal to the sum of Mg2+ and Ca2+ molalities with 1% excess (2.3851 mol/kg), which resulted in a decrease in the concentration of Mg2+ and Ca2+ to ~10−5 wt. % (Table 2). During the leaching of the model solution with Na2CO3, the concentration of NaCl exceeds its solubility limit, and it also starts to precipitate, which explains the decrease of Cl− molality during the treatment. It should also be noted that the molality of Li+ remains the same during the leaching, but the weight concentration increases due to a decrease in the density and total weight of the solution.PDF Image | Process of Lithium Recovery from Geothermal Brine
PDF Search Title:
Process of Lithium Recovery from Geothermal BrineOriginal File Name Searched:
membranes-11-00175-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |