
PDF Publication Title:
Text from PDF Page: 010
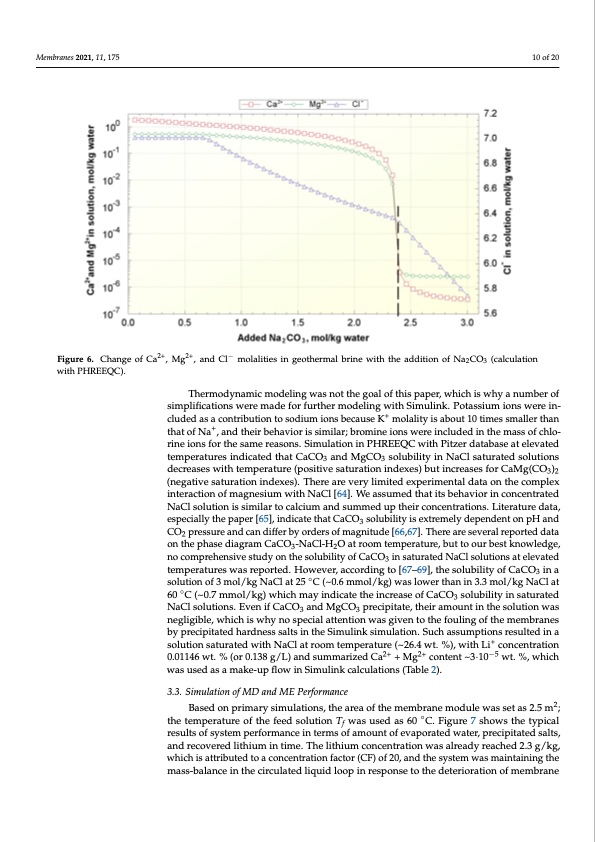
Membranes 2021, 11, 175 molalities with 1% excess (2.3851 mol/kg), which resulted in a decrease in the con- centration of Mg2+ and Ca2+ to ~10−5 wt. % (Table 2). During the leaching of the model solution with Na2CO3, the concentration of NaCl exceeds its solubility limit, and it also starts to precipitate, which explains the decrease of Cl− molality during the treatment. It should also be noted that the molality of Li+ remains the same during the leaching, but the weight concentration increases due to a decrease in the density and total weight of the solution. Figure 6. Change of 2C+a2+, M2g+2+, and C−l− molalities in geothermal brine with the addition of Na2CO3 (calculation Figure 6. Change of Ca , Mg , and Cl molalities in geothermal brine with the addition of Na2CO3 (calculation 10 of 20 with PHREEQC). with PHREEQC). Thermodynamic modeling was not the goal of this paper, which is why a num- Thermodynamic modeling was not the goal of this paper, which is why a number of ber of simplifications were made for further modeling with Simulink. Potassium simplifications were made for further modeling with Simulink. Potassium ions were in- ++ cludioednsaws aerceoinntcrilbuudteiodnatsoascoodniutrmibuiotinosnbteocasuodseiuKm imonolsablietycaiussaebKoutm1o0latilmityesismabaolluetr1th0an ++ thattiomfeNsasm,anllderththeairnbtehhaatvoiforNiass,iamnidlatrh;ebirobmehinaevionrsiswseimreilianrc;lubrdoemdinethioenmswasesroefinch-lo- rineciloundsedfoirnththeesammasesreoafscohnlos.riSniemiuolnastifonr itnhePHsaRmEeErQeaCsownist.hSPimitzuelratdioantaibnasPeHaRt EelEeQvaCted temwpeitrhatPuirtezserindaitcabteadsethaatteCleavCatOedatenmdpMergaCtuOresoinludbicilaiteydinthNataClasCaOtu3ranteddMsoglCutOio3ns 33 decrseoalusebsilwityitihntNemaCplersatureat(epdosoitliuvteiosnastudreactrioeansiensdwexitehs)tebmupt einracrtuearese(spfoosritCivaeMsagt(uCrOa-3)2 (negtiaotniviensdaetxuersa)tbiountincdrexaesse)s.fTohreCraeMarge(CveOr3y)2l(imneigteadtiveexpsaetruimraetniotnalinddateaxeosn).tThheecroemarpelex intevraecrytiolinmoitfemdaegxpnersimumenwtailtdhaNtaaoCnl t[h64e]c.oWmepalesxsuinmterdacthtiaotnitosfbmeahganveiosiruimn cwointhceNntarCatled NaC[6l4s]o.lWutieoansissusmimedilathratot ictaslcbieuhmavainordisnucmomncednturpatethdeNiracConlcseonlutrtaiotinonis.siLmitielararttuorecadla-ta, especciuiamllyatnhde psaupmemr [e6d5],uipndtihceaitre cthoantceCnatCraOtiosnosl.uLbiltietryaitsuerextrdeamtae,lyesdpepceianldlyentthoenppaHpearnd 3 CO pressureandcandifferbyordersofmagnitude[66,67].Thereareseveralreporteddata 2[65], indicate that CaCO3 solubility is extremely dependent on pH and CO2 pressure on the phase diagram CaCO -NaCl-H O at room temperature, but to our best knowledge, and can differ by orders3of magni2tude [66,67]. There are several reported data on nocomprehensivestudyonthesolubilityofCaCO insaturatedNaClsolutionsatelevated the phase diagram CaCO3-NaCl-H2O at ro3om temperature, but to our best temperatureswasreported.However,accordingto[67–69],thesolubilityofCaCO ina knowledge, no comprehensive study on the solubility of CaCO3 in saturated Na3Cl solution of 3 mol/kg NaCl at 25 ◦C (~0.6 mmol/kg) was lower than in 3.3 mol/kg NaCl at solutions at elevated temperatures was reported. However, according to [67–69], 60◦C(~0.7mmol/kg)whichmayindicatetheincreaseofCaCO solubilityinsaturated the solubility of CaCO3 in a solution of 3 mol/kg NaCl at 25 °C3 (~0.6 mmol/kg) was NaClsolutions.EvenifCaCO andMgCO precipitate,theiramountinthesolutionwas lower than in 3.3 mol/kg N3aCl at 60 °C3(~0.7 mmol/kg) which may indicate the in- negligible, which is why no special attention was given to the fouling of the membranes crease of CaCO3 solubility in saturated NaCl solutions. Even if CaCO3 and MgCO3 by precipitated hardness salts in the Simulink simulation. Such assumptions resulted in a solution saturated with NaCl at room temperature (~26.4 wt. %), with Li+ concentration 0.01146 wt. % (or 0.138 g/L) and summarized Ca2+ + Mg2+ content ~3·10−5 wt. %, which was used as a make-up flow in Simulink calculations (Table 2). 3.3. Simulation of MD and ME Performance Based on primary simulations, the area of the membrane module was set as 2.5 m2; the temperature of the feed solution Tf was used as 60 ◦C. Figure 7 shows the typical results of system performance in terms of amount of evaporated water, precipitated salts, and recovered lithium in time. The lithium concentration was already reached 2.3 g/kg, which is attributed to a concentration factor (CF) of 20, and the system was maintaining the mass-balance in the circulated liquid loop in response to the deterioration of membranePDF Image | Process of Lithium Recovery from Geothermal Brine
PDF Search Title:
Process of Lithium Recovery from Geothermal BrineOriginal File Name Searched:
membranes-11-00175-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |