
PDF Publication Title:
Text from PDF Page: 008
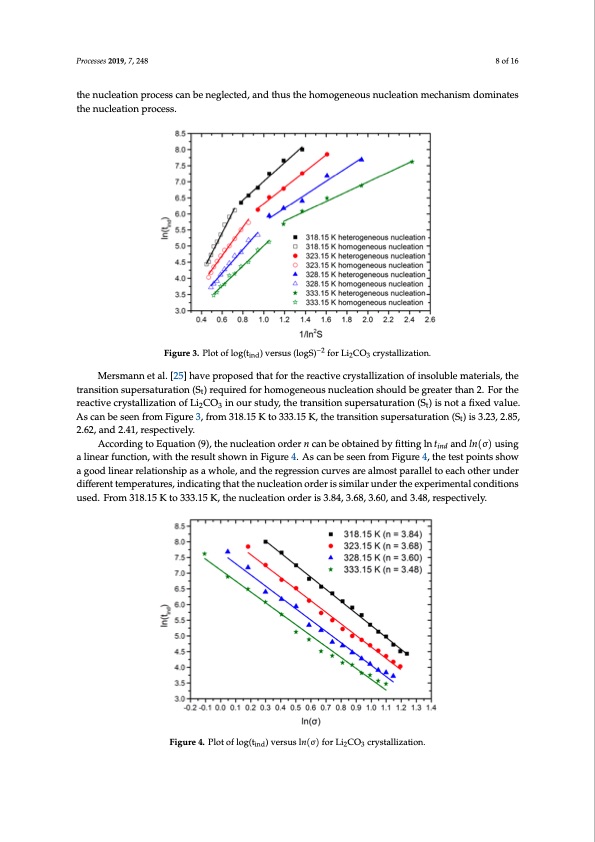
Processes 2018, 6, x FOR PEER REVIEW 8 of 17 reported for inorganic salts by other researchers [23,24]. This phenomenon can be attributed to a change in nucleation mechanism. At lower supersaturation levels, the driving force of the phase tPrraoncessietsio2n019i,s7,l2o48wer and the nucleation process is easily affected by external particles, so8otfh1e6 heterogeneous nucleation mechanism plays a leading role in the nucleation process. At higher supersaturation levels, the driving force of phase transition is larger. Compared with the spontaneous the nucleation process can be neglected, and thus the homogeneous nucleation mechanism dominates nucleation of solution, the influence of external particles on the nucleation process can be neglected, the nucleation process. and thus the homogeneous nucleation mechanism dominates the nucleation process. Figure 3. Plot of log(tind) versus (logS)−2 for Li2CO3 crystallization. Figure 3. Plot of log(tind) versus (logS)−2 for Li2CO3 crystallization. Mersmann et al. [25] have proposed that for the reactive crystallization of insoluble materials, the Mersmann et al. [25] have proposed that for the reactive crystallization of insoluble materials, transition supersaturation (St) required for homogeneous nucleation should be greater than 2. For the the transition supersaturation (St) required for homogeneous nucleation should be greater than 2. For reactive crystallization of Li2CO3 in our study, the transition supersaturation (St) is not a fixed value. the reactive crystallization of Li2CO3 in our study, the transition supersaturation (St) is not a fixed As can be seen from Figure 3, from 318.15 K to 333.15 K, the transition supersaturation (St) is 3.23, 2.85, value. As can be seen from Figure 3, from 318.15 K to 333.15 K, the transition supersaturation (St) is 2.62, and 2.41, respectively. 3.23, 2.85, 2.62, and 2.41, respectively. According to Equation (9), the nucleation order n can be obtained by fitting ln tind and ln(σ) using According to Equation (9), the nucleation order 𝑛 can be obtained by fitting ln𝑡 and 𝑙𝑛(σ) a linear function, with the result shown in Figure 4. As can be seen from Figure 4, the test points show using a linear function, with the result shown in Figure 4. As can be seen from Figure 4, the test points a good linear relationship as a whole, and the regression curves are almost parallel to each other under show a good linear relationship as a whole, and the regression curves are almost parallel to each other different temperatures, indicating that the nucleation order is similar under the experimental conditions uPnrodcesrsesd2i0ff1e8r, e6,nxtFtOemR PpEeErRatRuErVeIsE,Windicating that the nucleation order is similar under the experim9enofta1l7 used. From 318.15 K to 333.15 K, the nucleation order is 3.84, 3.68, 3.60, and 3.48, respectively. conditions used. From 318.15 K to 333.15 K, the nucleation order is 3.84, 3.68, 3.60, and 3.48, respectively. Figure 4. Plot of log(t ) versus ln(σ) for Li CO crystallization. Figure 4. Plot of log(tiind) versus l𝑛(σ) for Li2CO3 crystallization. 3.1.3. Identification of Crystal Growth Mechanism The growth-mechanism of Li2CO3 crystals was identified by fitting the experimental induction times from reactive crystallization over a range of temperatures and supersaturations to the expressions of different growth mechanisms. According to a previous study [26], Li2CO3 crystals are rod-like, meaning the value of m is 1. Hence n (mν + 1) can take values of either 3/2 or 2 depending on the value of ν (1/2 or 1). The expressions Fu(S) for different growth mechanisms of Li2CO3 arePDF Image | Reactive Crystallization Process of Lithium Carbonate
PDF Search Title:
Reactive Crystallization Process of Lithium CarbonateOriginal File Name Searched:
processes-07-00248-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |